Monoclonal antibody against human carboxypeptidase a inhibitor latexin and its application
A monoclonal antibody and antibody technology, applied in the direction of anti-animal/human immunoglobulin, biochemical equipment and methods, instruments, etc., can solve problems such as the complex mechanism of tumor cells
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
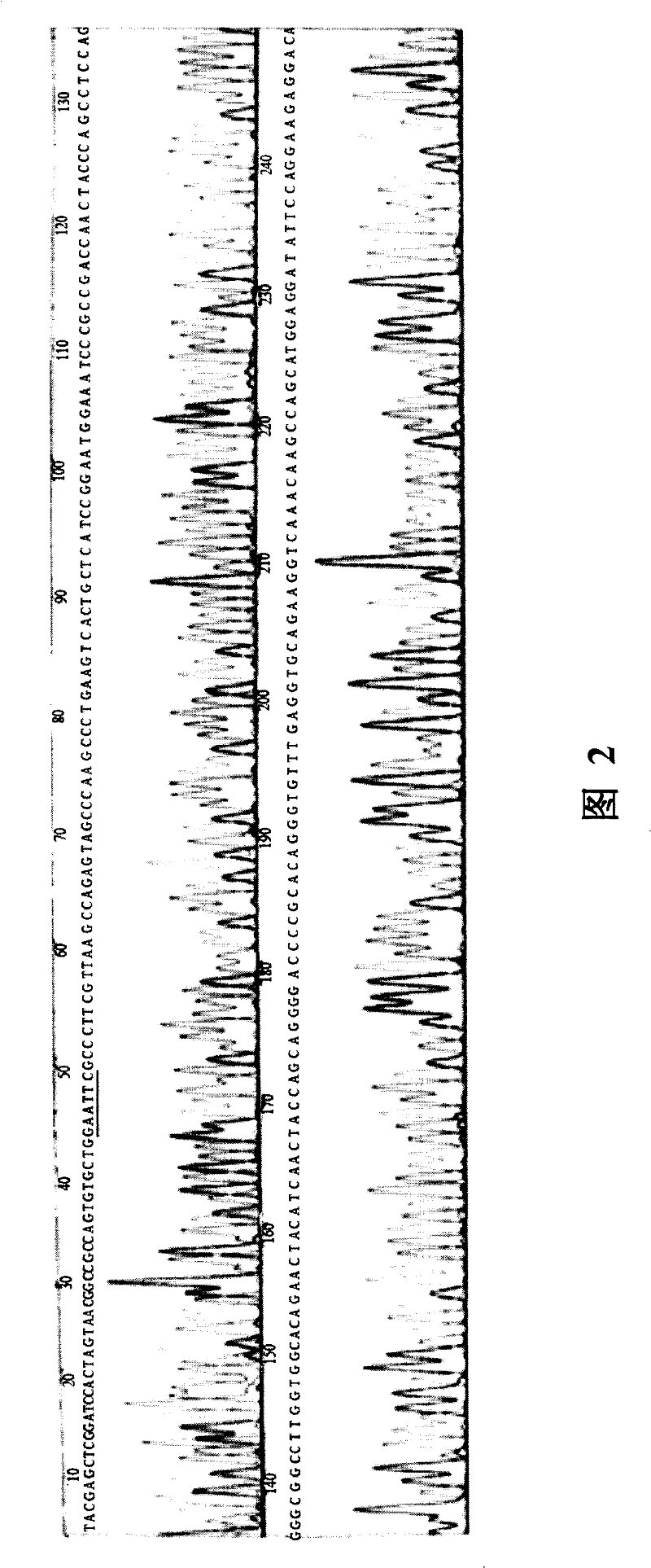
[0055] Example 1. Acquisition and identification of the full-length reading frame sequence of Latexin gene
[0056] 1. RACE method to amplify the 5′ end of Latexin
[0057] 1. Preparation of total cellular RNA: use Trizol LS Reagent (Cat. No. 10296-010), a product of Invitrogene Company, and operate according to the product instruction.
[0058] 2. Use SMART TM RACE cDNA amplification kit (purchased from Clontech Company) to obtain Latexin 5' end fragments:
[0059]2.1 Synthesis of the first strand of cDNA
[0060] 10mM Poly Oligo * (dT) 1μl
[0061] Total cellular RNA 3μl (1μg)
[0062] SMART II A Oligo ** (10mM) 1μl
[0063] ↓
[0064] 70°C for 5 minutes, ice bath for 2 minutes
[0065] ↓
[0066] 5×first strand synthesis solution 2μl
[0067] DTT (20mM) 1μl
[0068] dNTP (10mM) 1μl
[0069] PowerScript TM Reverse transcriptase 1 μl
[0070] ↓
[0071] 1.5 hours at 42°C
[0072] ↓
[0073] When terminating the reaction, add 100ml Tricine-EDTA buffer soluti...
Embodiment 2
[0094] Example 2. Construction of Latexin prokaryotic expression vector pGEX / Latexin, prokaryotic expression and purification of antigen
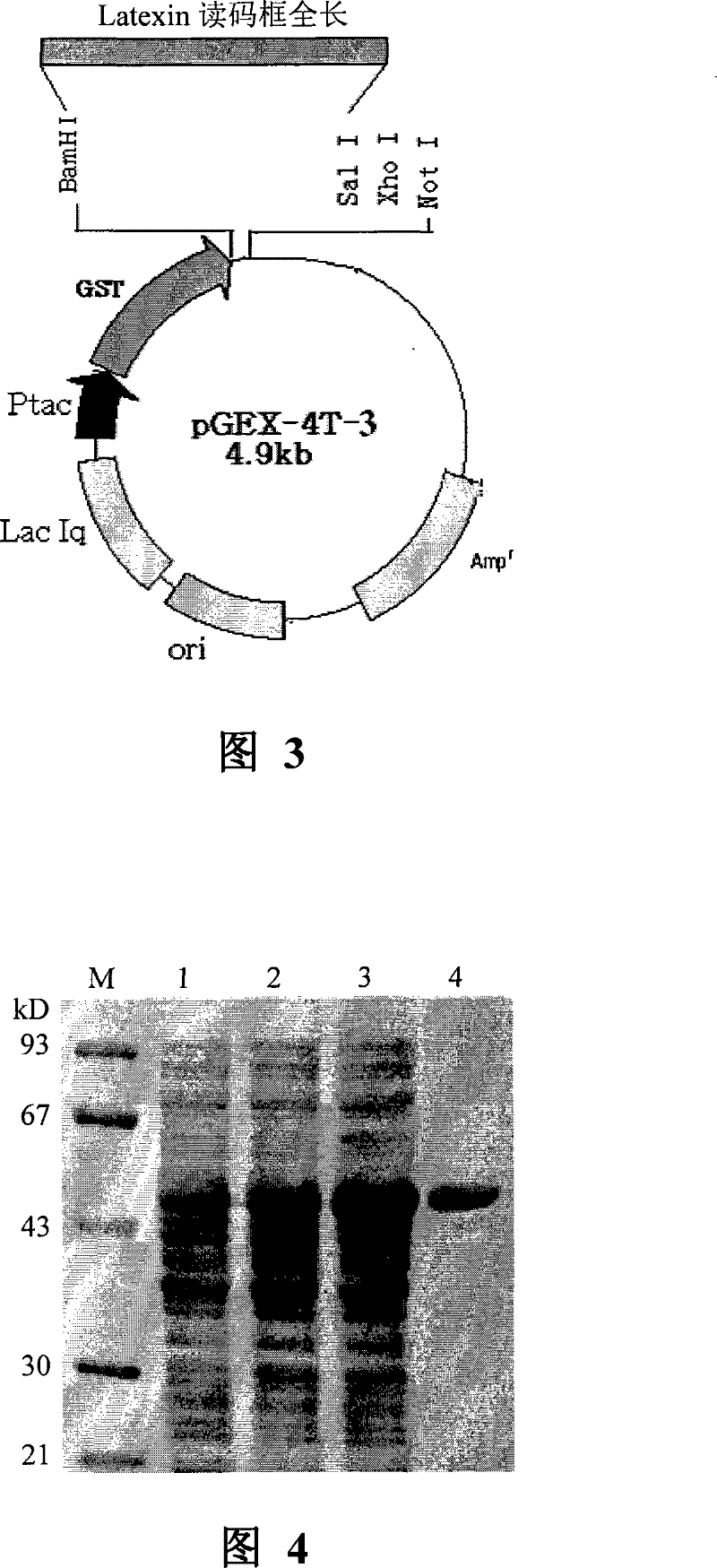
[0095] 1. Construction of Latexin prokaryotic expression vector pGEX / Latexin
[0096] pcDNA3-Latexin was digested with EcoRI, recovered and purified to cut out a 756bp fragment (Latexin cDNA194-887 (positions 194-887 shown in SEQ ID No. 1), in addition to containing a small sequence from the pcDNA3 vector), which Clone into pGEX-4T-3 (purchased from Promega) to construct a prokaryotic expression vector of pGEX-4T-3-Latexin, transform it into BL21 expression bacteria, and spread LB Amp+ Disk, clone picking, plasmid extraction, enzyme digestion identification, automatic sequencing, identification of inserts and reading frames, and construction of prokaryotic expression plasmid pGEX-Latexin fusion protein.
[0097] 2. Induction, expression and purification of GST-Latexin recombinant protein
[0098] 1. Aseptically draw 5ml of pGEX-4T-3-Latex...
Embodiment 3
[0117] Example 3. Preparation, purification and identification of monoclonal antibody against human carboxypeptidase A inhibitor Latexin
[0118] 1. Animals: female, 4 Balb / c mice aged 6-8W (purchased from Institute of Experimental Animals, Chinese Academy of Medical Sciences), 18-20g / mouse.
[0119] 2. Antigen: GST-Latexin fusion protein is expressed by prokaryotic, purified by SDS-PAGE gel, the purity reaches the electrophoresis grade, 50μg / animal / time.
[0120] 3. Immunization procedures and approaches:
[0121] First immunization: Latexin antigen was fully mixed with an equal volume of complete Freund's adjuvant to form water-in-oil, and injected intradermally at multiple points on the back of the mouse. Four weeks later, the first booster immunization was performed. Latexin antigen was mixed with incomplete Freund's adjuvant in equal volumes, and injected intradermally at multiple points on the back. Afterwards, immunization was boosted once every other month, and 2 μl ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com