Fenofibrate medicament composition
A technology for fenofibrate and a composition, applied in the field of fibrate drugs, can solve problems such as low bioavailability, and achieve the effects of stable preparation means, simple production process, and reducing differences in bioavailability
Inactive Publication Date: 2010-11-10
安徽省食品药品检验研究院
View PDF5 Cites 5 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
In order to solve the problem of low bioavailability of fibrates represented by fenofibrate, the present invention provides a fibrate pharmaceutical composition that significantly improves in vitro dissolution rate and bioavailability
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
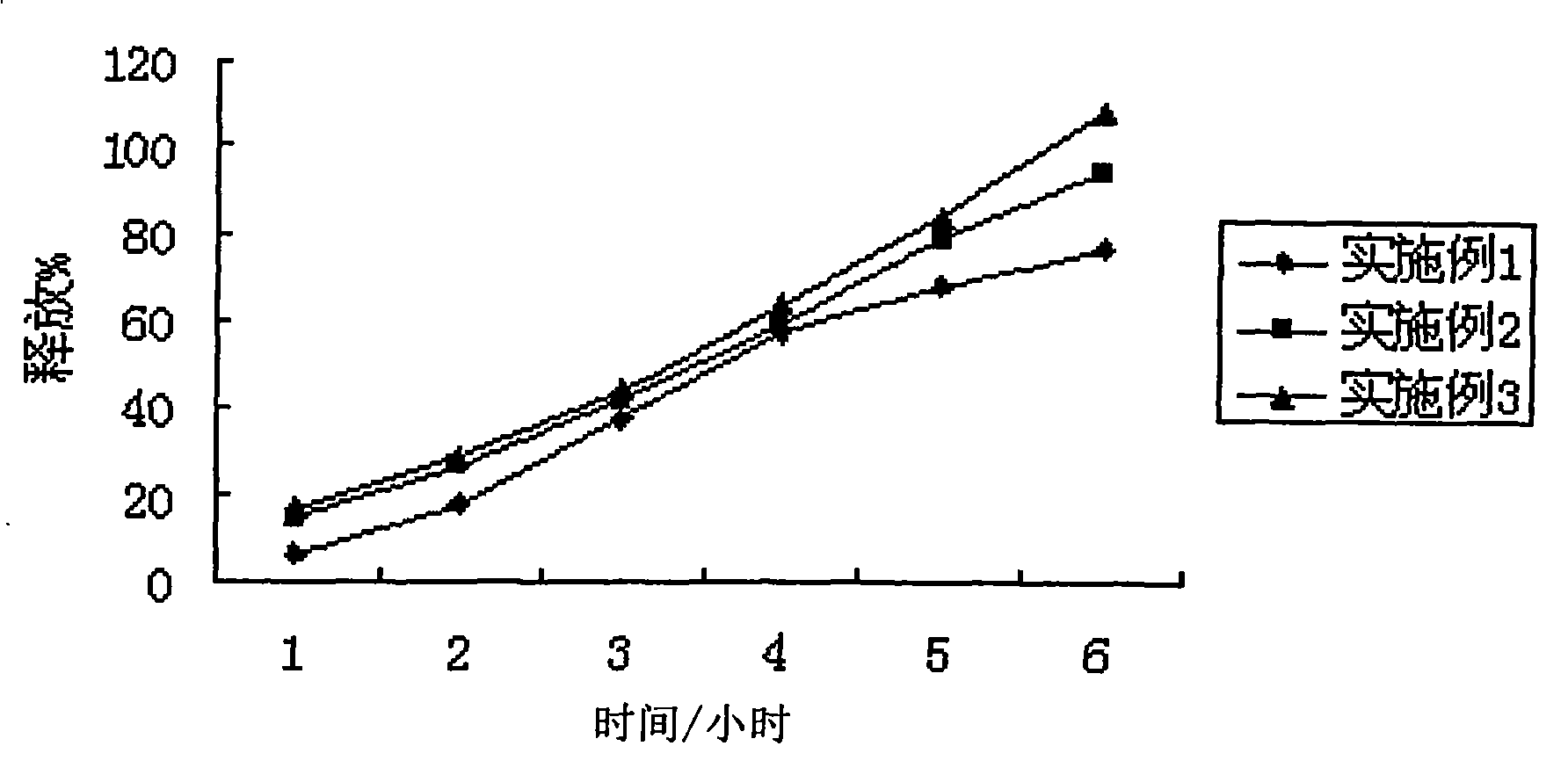
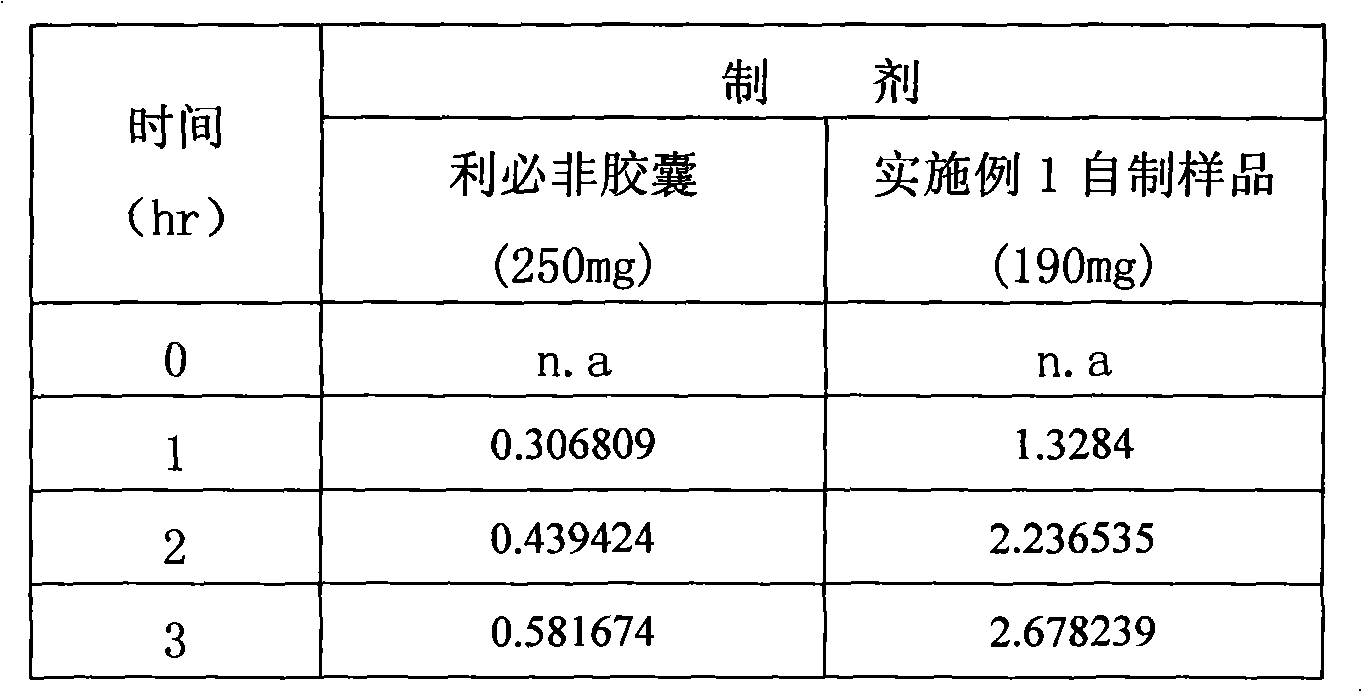
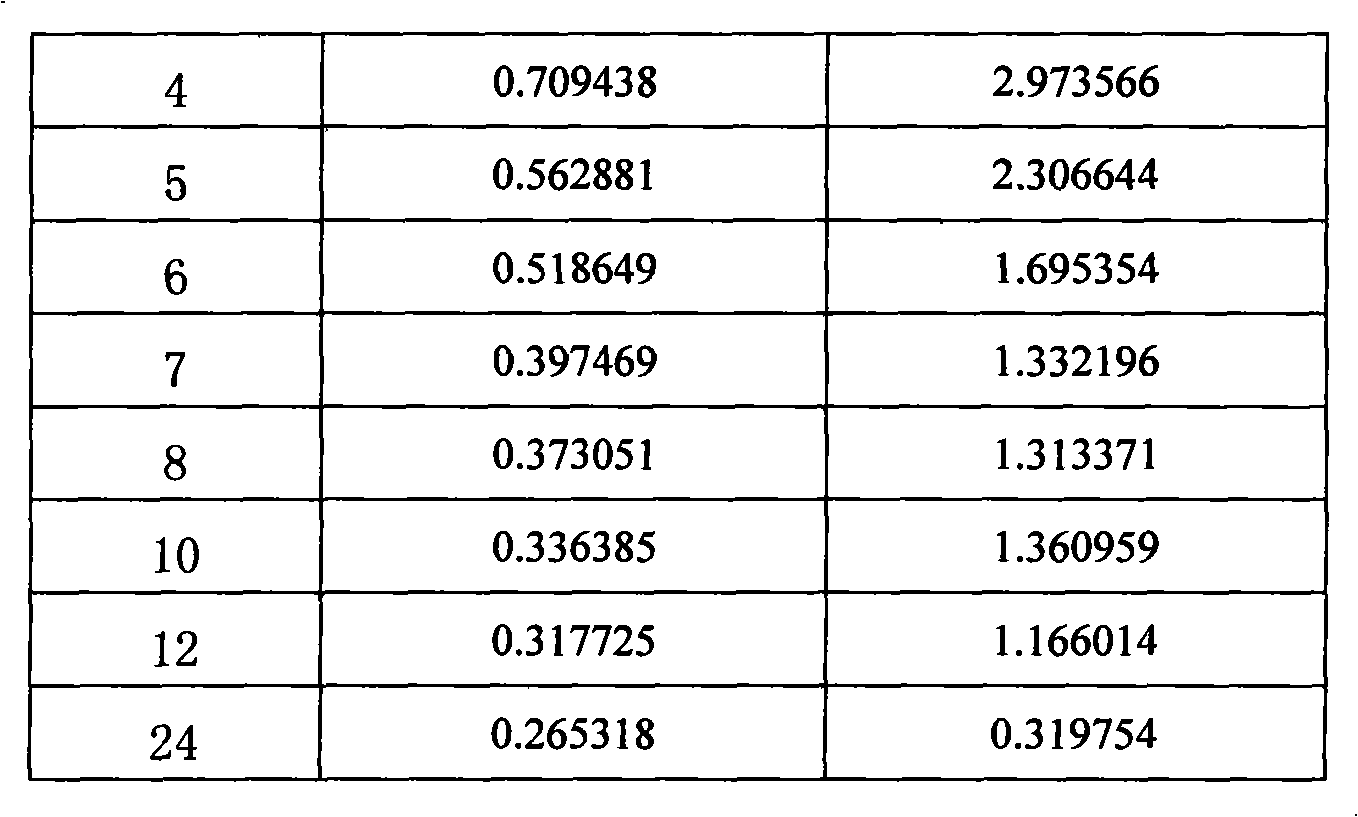
Embodiment 1
Embodiment 2
Embodiment 3
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
| Property | Measurement | Unit |
|---|---|---|
| degree of polymerization | aaaaa | aaaaa |
| degree of polymerization | aaaaa | aaaaa |
Login to View More
Abstract
The invention relates to a fibrate drug combination and solves the problem of the low bioavailability of fenofibrate. The drug combination comprises the following materials according to part by weight: 6 to 42 parts of fenofibrate, 60 to 80 parts of polyethylene glycol 6000 or poloxamer 407, 6 to 42 parts of compritol 888 ATO or precirol AT05, 2 to 12 parts of polyethylene glycol glyceryl dilaurate or ethylene glycol monoethyl ether and 2 to 12 parts of cremophor RH40 or cremophor EL. The invention can finish the processes of drug micronization and micronized drug dispersion in one-step operation based on the physical and chemical properties of raw materials and auxiliary materials; the compritol 888 ATO can achieve the purpose of sustaining the drug release owing to the hydrophobicity thereof, and the compritol 888 ATO has the release characteristics of zero-order sustained release at a particular ratio in a continuous and complete state; the hydrophilic matrix formed by polyethyleneglycol 6000 can prevent the drug release from being affected by acid and alkali and reduce the possibility of bioavailability difference caused by the individual difference; and the invention has theadvantages of simple production process, low cost and controllable quality.
Description
Fenofibrate pharmaceutical composition technical field The invention relates to fibrate drugs (selected from phenoxyaromatic acid drugs, salts thereof, solvates and active metabolites thereof), specifically a stable solid dosage form of fenofibrate and a preparation method thereof. Background technique Fenofibrate is a second-generation phenoxyaromatic acid drug developed by French company Fournier and launched in the United States in 1998. Because of its good lipid-lowering effect, it has been widely used in clinical practice. Fenofibrate has a good curative effect, but because it is insoluble in water, the dissolution rate is insufficient, resulting in low bioavailability after oral administration. In the digestive tract, it is only about 60% absorbed after oral administration. Its bioavailability is incomplete, and varies from person to person, with large individual differences. Generally required to be used with food to enhance bioavailability. Therefore, if the ...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More Patent Type & Authority Patents(China)
IPC IPC(8): A61K9/48A61K9/20A61K31/216A61K47/44A61P3/06A61K47/34A61K47/14A61K47/10
Inventor 刘羽王辉吕凌孙备王贺路小冬李姜晖刘燕戴萍萍崔颖
Owner 安徽省食品药品检验研究院
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com