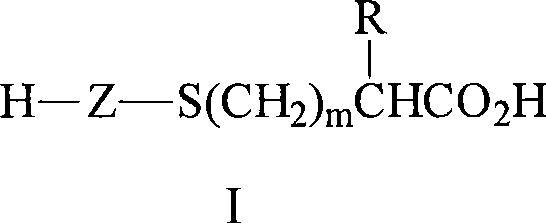Fluorinated surfactants and method of making the same
A technology of surfactant and fluorination, which is applied in the field of fluorinated surfactant and its preparation, and can solve problems such as reducing the surface tension of solvents or preparations
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example
[0160] All parts, percentages, ratios, etc. in the examples and elsewhere in the specification are by weight unless otherwise indicated. The ratios of N-methylperfluorobutanesulfonamidoethyl acrylate (MeFBSEA) to -C(O)OH and -C(O)O- groups reported in the examples below are based on the amount of starting monomer molar ratio.
[0161] In the following examples, MeFBSEA was prepared according to the method in Parts A and B of Example 2 of U.S. Patent No. 6,664,354 (Savu), which is incorporated herein by reference, except that 4270 kg of N-methylperfluorobutanesulfonamidoethanol, 1.6kg of phenothiazine, 2.7kg of methoxyhydroquinone, 1590kg of heptane, 1030kg of acrylic acid, 89kg of methanesulfonic acid (instead of trifluoromethanesulfonic acid ) and 7590kg of water.
[0162] Weight Average Molecular Weight Determination
[0163] The weight average molecular weights in Examples 1-10, 12 and 17 were determined by comparison to linear polystyrene polymer standards using gel p...
example 1
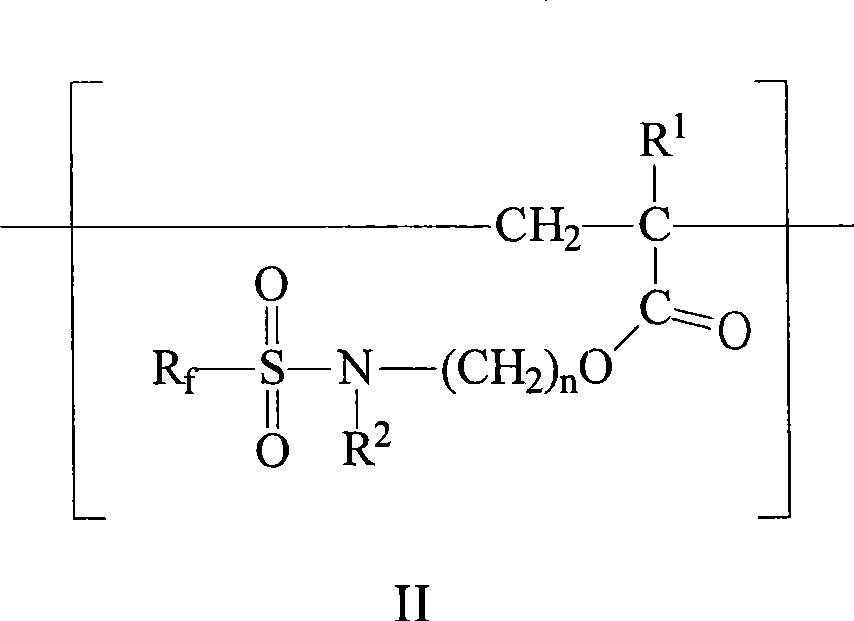
[0167] In a 500-mL flask, blend MeFBSEA (37 grams (g), 0.09 moles (mol)), acrylic acid (4.3 g, 0.06 mol) and mercaptosuccinic acid (4.5 g, 0.03 mol) with isopropanol (IPA) The mixture was diluted to approximately 50% solids. Add 2,2′-azobis(2-methylpropionitrile) (AIBN) (0.1%), use vacuum and nitrogen degassing to react three times, and heat at 70° C. under nitrogen atmosphere for six hours. Additional AIBN (0.05%) was added and heating continued at 70°C for an additional 16 hours. The weight average molecular weight of the polymer was determined to be 1800 by GPC. MeFBSEA with -C(O)OH and -C(O)O - The group ratio was 0.75.
[0168] The reaction was diluted to 30% solids with IPA. Dimethylethanolamine (DMEOA) (12.6 g, 0.12 mol) was added to neutralize the acid and the mixture was further diluted to 25% solids with deionized water. At 25% solids and 50°C, the mixture was visually determined to be a single phase clear and stable solution.
[0169] Deionized water was added...
example 2-6
[0171] Examples 2-6 were prepared as described in Example 1 except that the reagents and amounts indicated in Table 1 (below) were used.
[0172] Table 1
[0173] example
[0174] At 25% solids and 50°C, the mixtures containing the neutralized oligomers of Examples 2, 5 and 6 were visually determined to be single-phase clear and stable solutions, while the mixtures containing the neutralized oligomers of Examples 3 and 4 The mixture was a single-phase cloudy solution. The weight average molecular weights of the oligomers were determined by GPC and are listed in Table 1 (above).
PUM
| Property | Measurement | Unit |
|---|---|---|
| Weight average molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com