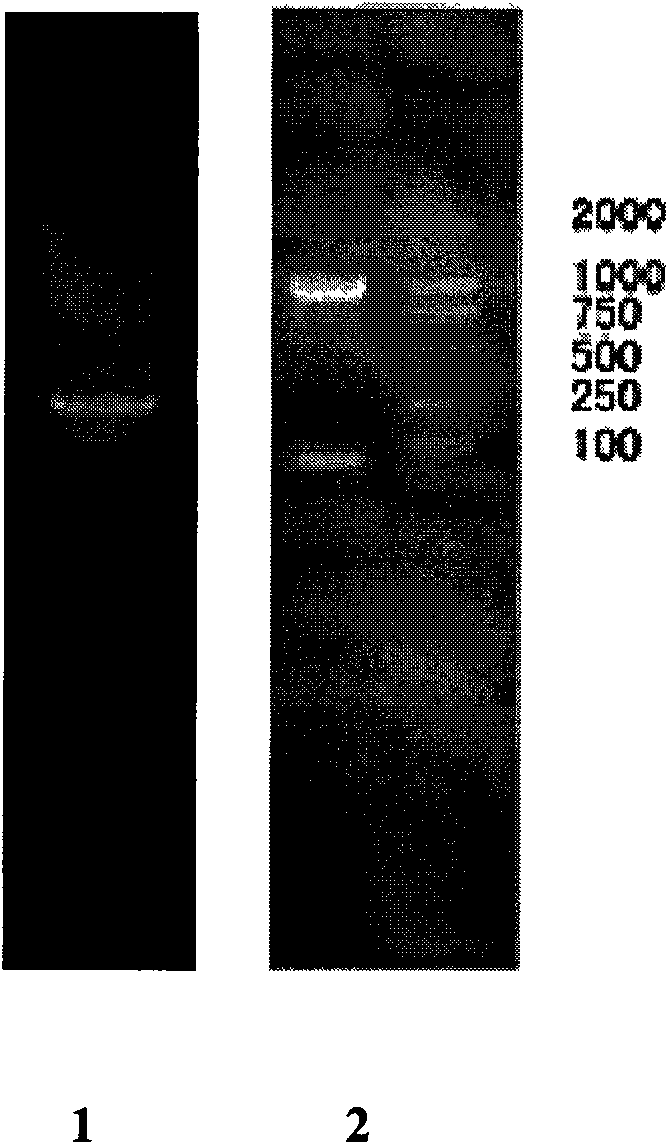DNA vaccine for preventing and treating cysticercosis cellulosae and preparation method thereof
A DNA vaccine, a technology for swine cysticercosis, which can be used in gene therapy, pharmaceutical formulations, genetic material components, etc., can solve the problems of poor cell line stability, large side effects, and unsatisfactory curative effects, and achieve stable physical and chemical properties and low cost. , the effect of good immune protection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Example 1: Cloning of the Anx B2 gene from Hexacarpa
[0037]1. Activation of Taenia solium, extraction of total RNA and RT-PCR, PCR amplification
[0038] Fresh tapeworm proglottids collected from Taenia solium patients (purchased from Harbin Medical University) were rinsed several times with saline, cut into pieces in a 7ml Eppendorf tube and squeezed to release the eggs, centrifuged and rinsed 3 times at 3000r / min ; Add an equal amount of sodium hypochlorite to act for a few minutes (microscopic examination of most eggs broken), quickly add an equal amount of 0.2mol / L sodium thiosulfate to terminate the reaction, centrifuge at 3000r / min to remove egg fragments; resuspend with normal saline Precipitate, add hatching activation solution containing trypsin and pig bile, incubate at 37°C for 40 minutes (violently shake once every 10 minutes), centrifuge to discard the supernatant, and the precipitate is the activated Hexacarpa. Weigh 30 mg of Hexacarpa precipitate and p...
Embodiment 2
[0045] Example 2: Construction and preparation of pCDNA3.1-Anx B2 vaccine
[0046] 1. Construction of pCDNA3.1-Anx B2 DNA vaccine
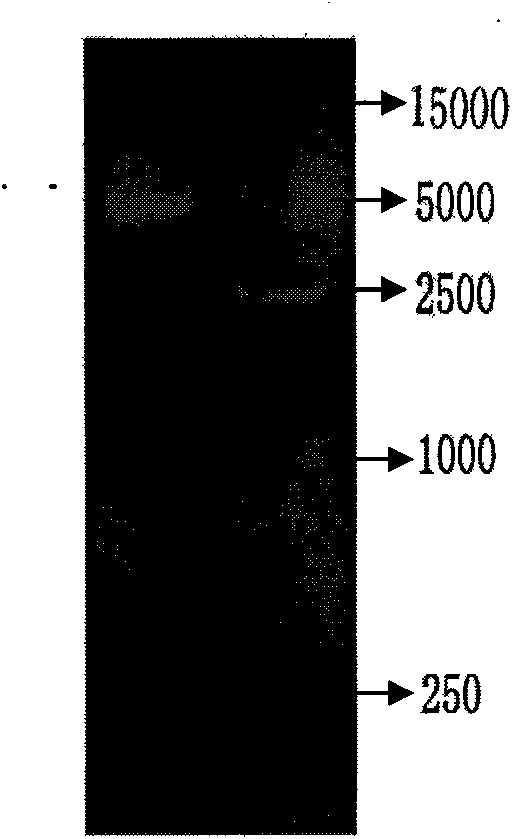
[0047] Using the plasmid pGEM-T-Anx B2 containing the target gene as a template, PCR amplification was carried out with the above-mentioned primers 1 and 2. After the PCR product was recovered from the gel, it was double-digested with BamH I and xho1 (purchased from NEB Company), and the same BamH I and xho1 double-digested pCDNA3.1 vector (purchased from Invitrogen) was connected by T4 ligase, transformed into Escherichia coli DH5α, positive recombinants were obtained by blue-white screening, extracted plasmid, BamH I, xho1 digestion and sequencing identification, sequencing The result was correct, and the engineering bacteria DH5α containing the recombinant plasmid pCDNA3.1-Anx B2 was obtained. (For specific experimental steps, refer to the third edition of "Molecular Cloning", Science Press, published in 2002 for related content)
[0048] 2. ...
Embodiment 3
[0051] Example 3: In vitro expression of pCDNA3.1-Anx B2 vaccine
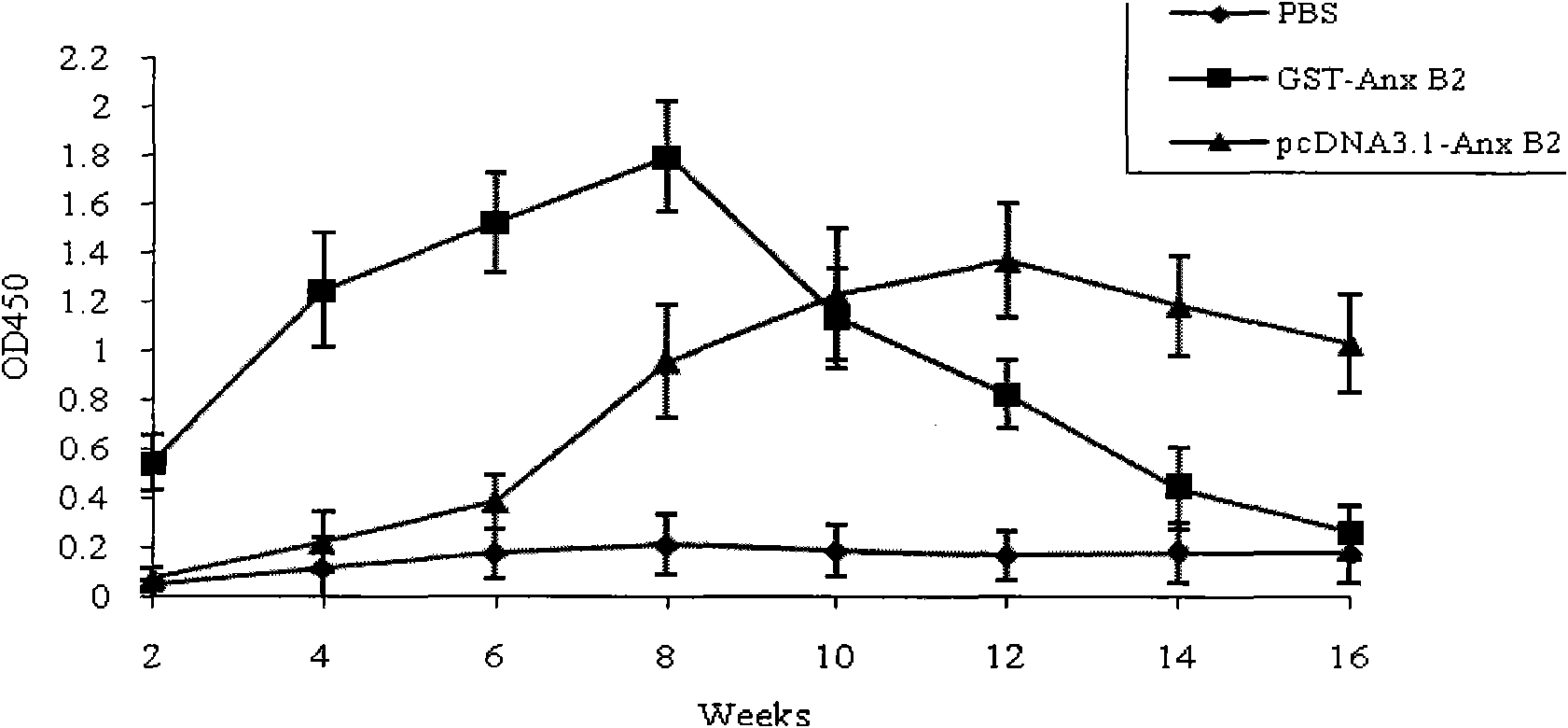
[0052] The constructed DNA vaccine was transfected into COS7 cells by electroporation (see the third edition of "Molecular Cloning" for specific steps, published by Science Press in 2002), and the cells were harvested after 72 hours, and detected by indirect ELISA. The expression level of the antigen Anx B2 in the supernatant and cell lysate was positive after color development, and the P / N ratio was greater than 1.8. The result was positive in the supernatant and cell lysate, and the AnxB2 antigen was expressed in COS7 cells. It shows that the DNA vaccine can be expressed in mammalian cells.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com