Antineoplastic medicine composition and application thereof
A technology for anti-tumor drugs and compositions, which can be used in anti-tumor drugs, drug combinations, pharmaceutical formulations, etc., and can solve problems such as no compounds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
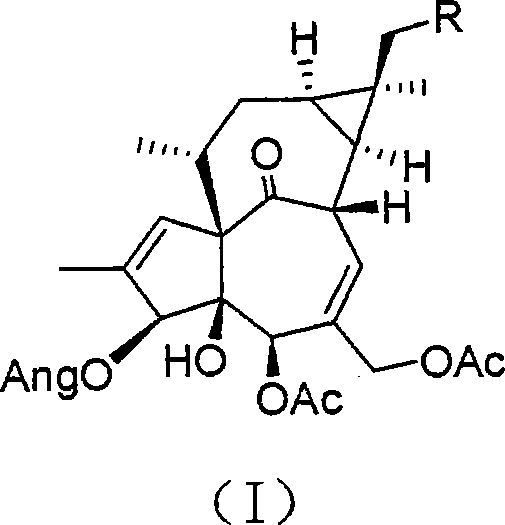
[0035] 1. Compound 1 (5,17,20-triacetyl-ingen-3-angelate) and compound 2 (5,20-diacetyl-ingen-3-angelate) preparation:
[0036] Take 10Kg (dry weight) of the above-ground part of Euphorbiaceae Euphorbia plant Bawangbian, crush it, extract it with 70% acetone water (20L×3), concentrate it under reduced pressure to a total volume of 2L, and extract it with ethyl acetate (2L×3) Finally, the ethyl acetate part was decolorized through an MCI column to obtain 120 g of medicinal extract. Eluted by silica gel column chromatography (petroleum ether-Me 2 CO=1:0~1:1) was divided into 5 parts A-E; part A was further separated by silica gel column chromatography (petroleumether-Me 2 CO=100:1) to obtain a total of 4 parts a-d; continue to use HPLC (Agilent1200HPLC system; Zorbax SB-C18, 250×9.4) to purify, part c was separated with 76% methanol and water to obtain 5,17,20-triacetyl - Ingen-3-angelate (3 mg).
[0037] Take 10Kg (dry weight) of the above-ground part of Euphorbiaceae Eupho...
Embodiment 2
[0045] Compound 1 (5,17,20-triacetyl-ingen-3-angelate) and compound 2 (5,20-diacetyl-ingen-3-angelate) on mice The impact of spontaneous metastasis of Lewis lung cancer:
[0046] Lewis lung cancer cell line was made into tumor cell suspension, and the trypan blue count was about 1×10 7 / mL tumor cells. Disinfect the right anterior armpit of the mouse, take 0.2mL (about 1×10 6 -2×10 6 Tumor cells) were inoculated subcutaneously. Passage 3 times. The subcutaneous tumor mass of Lewis lung cancer tumor-bearing mice was peeled off under sterile conditions, and the well-grown tumor tissue was selected, cut into pieces, weighed, made into a homogenate with a cell homogenizer, and added with normal saline at a volume ratio of 1:3 to make a tumor The cell suspension was inoculated subcutaneously in the armpit of the right forelimb of C57BL / 6J mice, each 0.2mL (about 1×10 6 ~2×10 6 tumor cells). The next day randomized grouping, compound 1 (5,17,20-triacetyl-ingen-3-angelate) an...
Embodiment 3
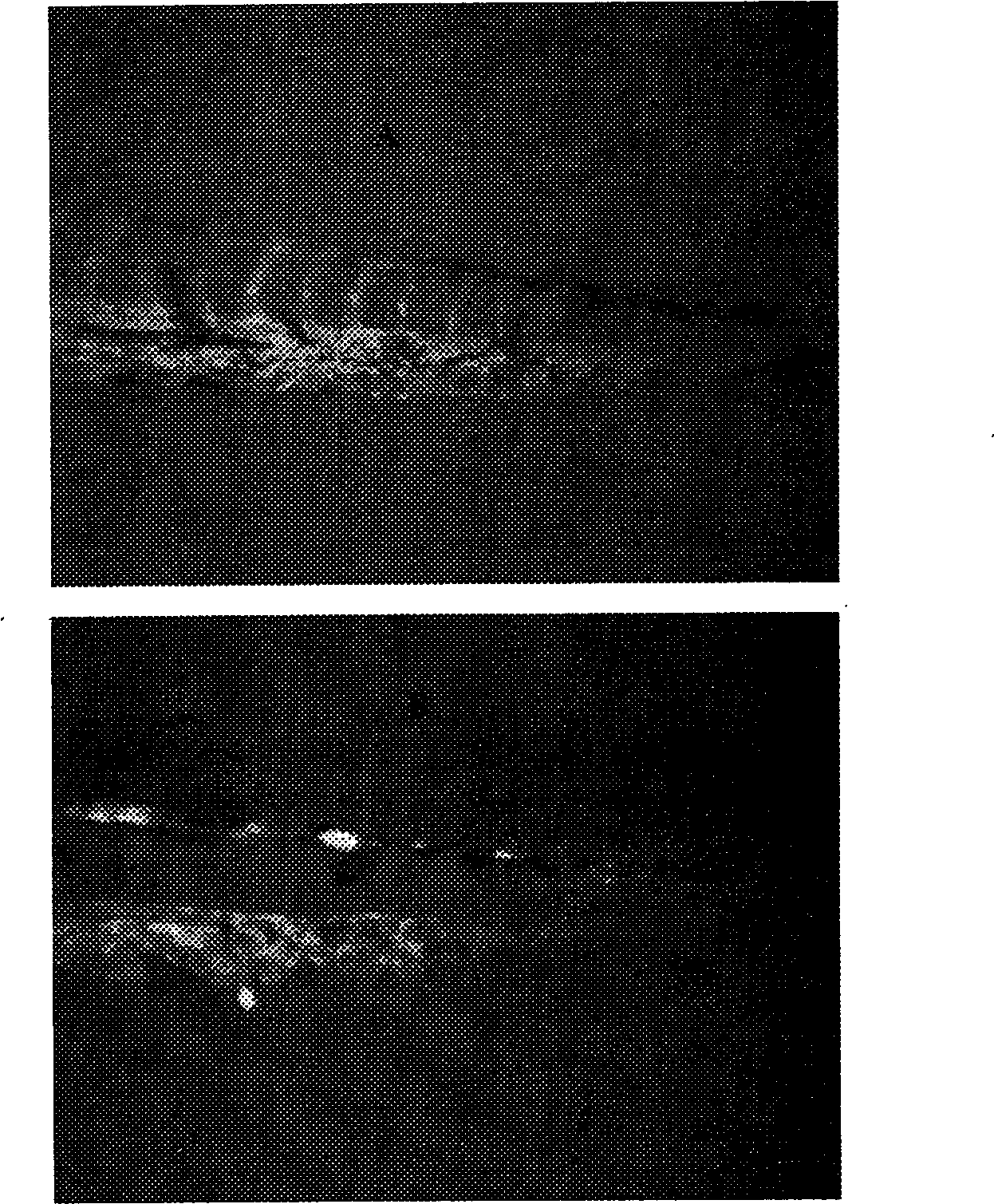
[0052] The effects of compound 1 (5,17,20-triacetyl-ingen-3-angelate) and compound 2 (5,20-diacetyl-ingen-3-angelate) on neovascularization generated inhibition
[0053] When the fertilized eggs of TG (VEGFR2:GFP) vascular fluorescence transgenic zebrafish were developed for 24 hours, normal zebrafish embryos were selected under a stereoscope and transferred to a 96-well culture plate, one embryo per well. Add a certain volume of medicine to the sample wells in advance, 32 wells per concentration, and then supplement the volume with nutrient water to 300 μl, and the control is embryo nutrient water. At 72 hours of fertilized egg development, the growth of zebrafish intersegmental blood vessels was observed with a fluorescence microscope, the inhibition rate was calculated, and the death of embryos or larvae was observed.
[0054] The vascular development process of zebrafish embryos is very similar to that of humans. The branches of the main blood vessels on the back of the z...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com