Method for synthesizing prasugrel intermediate and method for synthesizing prasugrel
A synthetic method and intermediate technology, applied in the chemical and pharmaceutical fields, can solve the problems of high synthesis cost, reduced reaction efficiency, low yield, etc., and achieve the effect of convenient operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
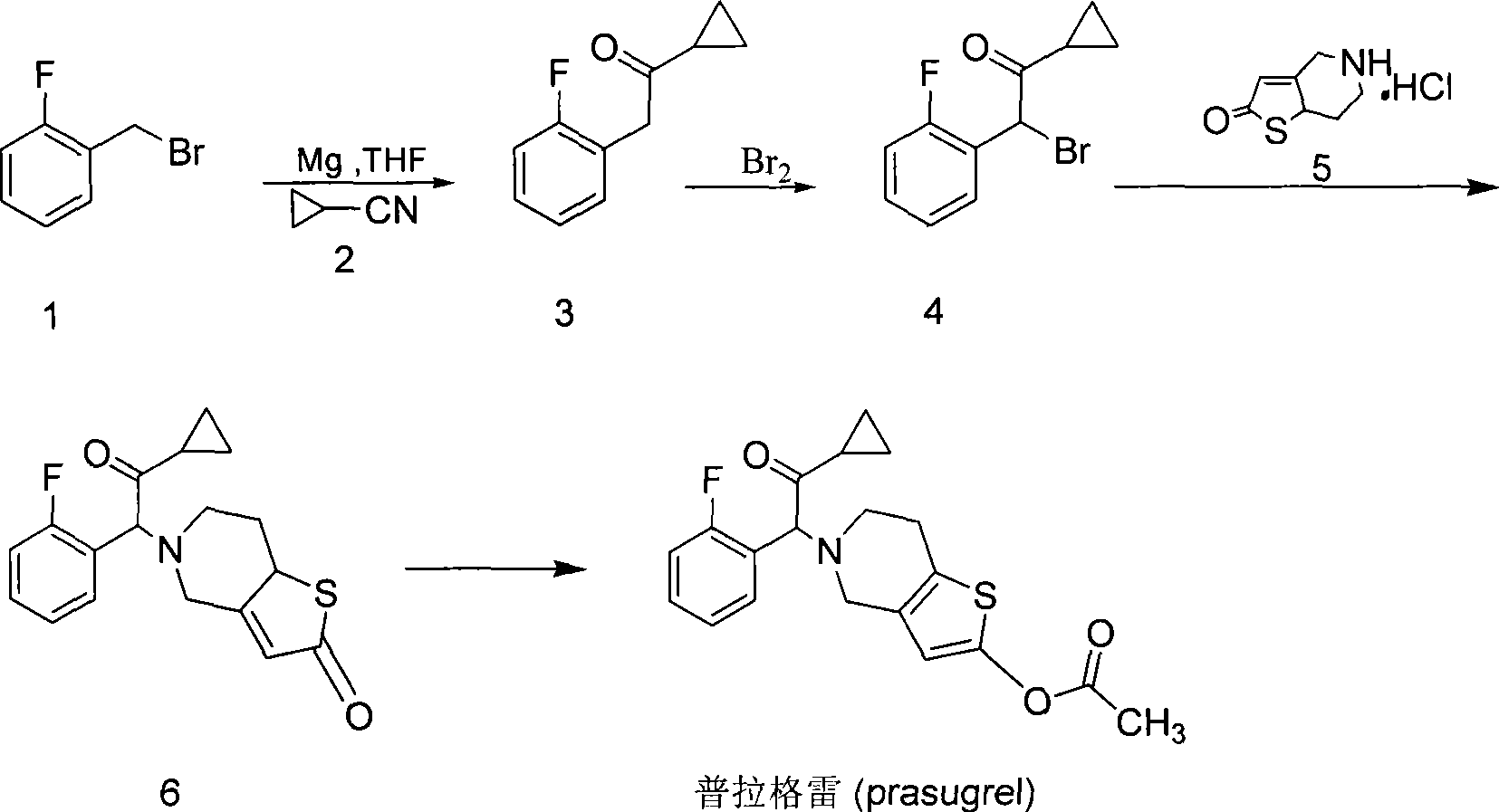
[0026] The present invention is further illustrated by the following examples, but the scope of the present invention is not limited.
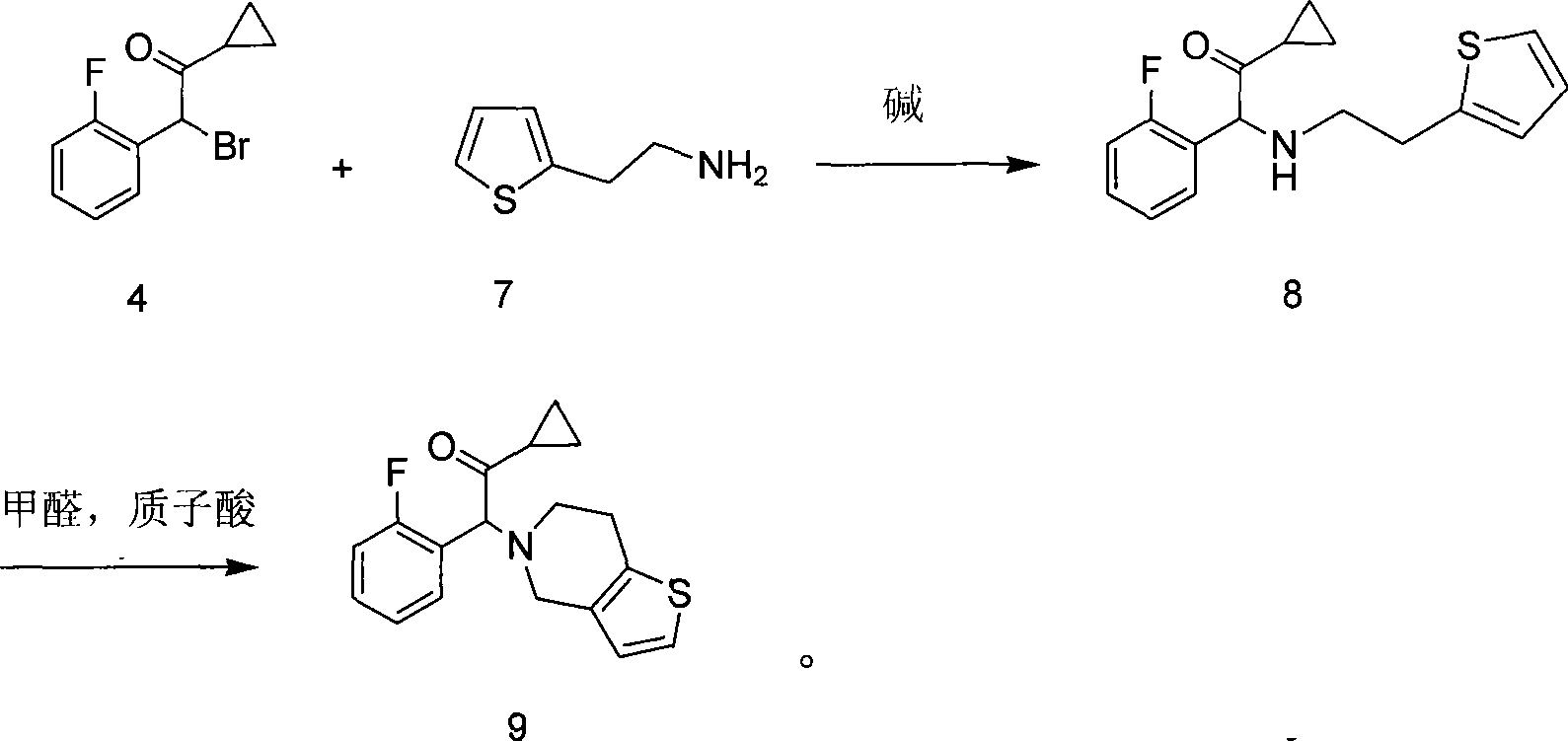
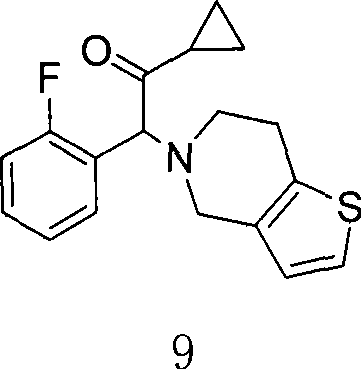
[0027] The first step: the synthesis of 1-cyclopropyl-2-(2-fluorophenyl)-2-(2-(thiophen-2-yl)ethylamine)ethanone, the reaction formula is:
[0028]
[0029] 2-Thienylethylamine (2.3g, 18.1mmol) was dissolved in 80ml of dichloromethane, potassium carbonate (2.5g, 18.1mmol) was added, and the 2 Slowly add 2-bromo-1-cyclopropyl-2-(2-fluorophenyl)ethanone (3.82g, 14.8mmol) in dichloromethane solution dropwise under protection. , washed with water (50ml×3), the organic layer was separated, dried with anhydrous sodium sulfate, suction filtered, and evaporated to dryness under reduced pressure. After separation by column chromatography, 3.14g of light yellow liquid was obtained, with a yield of 73%.
[0030] 1 H NMR (400MHz, CDCl 3 )δ: 0.72-1.08 (4H, m), 1.87 (1H, m), 2.75 (1H, m), 2.85 (1H, m), 3.00 (2H, m), 4.95 (1H, s), 6.79-7.30 (7H, m).ES...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com