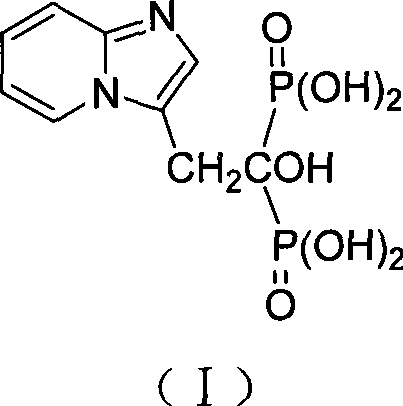
High-purity minodronic acid and preparation method thereof
A bisphosphonic acid and phosphorylation technology, which is applied to the field of high-purity minodronic acid and its preparation, can solve the problems of many reaction steps, difficult product purification, large environmental pollution, etc., and achieves reduction of reaction steps, difficulty reduction, The effect of strong reaction controllability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
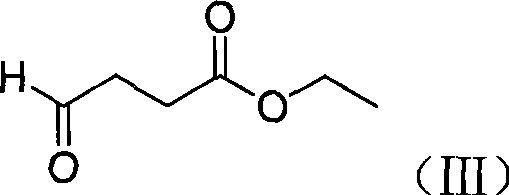
[0040] 1, the preparation of ethyl 4-formyl butyrate (III)
[0041] N 2 Under protection, succinic acid monoethyl chloride (16.5g, 0.1mol) was dissolved in dried THF (1L), cooled to -80°C, and LiAlH(OC 4 h 9 -t) THF (1L) solution of (38g, 0.15mol), dripping is completed, stirred at low temperature for 5h, the reaction is completed, the reaction solution is poured into a large amount of ice water, extracted with ethyl acetate (600ml×3), combined with ethyl acetate, Dry, filter, and concentrate to obtain 7.9 g of yellow liquid, with a yield of 61%.
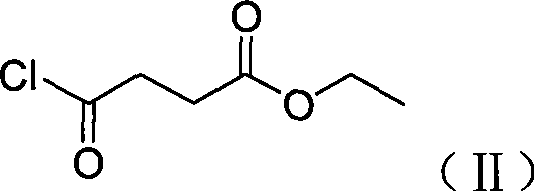
[0042] Preparation of 2.3-bromo-4-formyl butyric acid ethyl ester (IV)
[0043] Compound (III) (7.9g, 0.06mol) and trimethylchlorosilane (7.8g, 0.072mol) were added to the reaction flask, the temperature was lowered to 0°C, and triethylamine (6g, 0.06mol) was added dropwise. After dropping, the temperature was raised to reflux, and after 4 hours it was cooled to room temperature, 200ml of chloroform was added, and a solution of ...
Embodiment 2
[0051] 1, the preparation of ethyl 4-formyl butyrate (IV)
[0052] N 2 Under protection, succinic acid monoethyl chloride (16.5g, 0.1mol) was dissolved in dried DMF (1L), cooled to -80°C, and LiAlH(OC 4 h 9 -t) DMF (1L) solution of (38g, 0.15mol), dripping is completed, stirred at low temperature for 5h, the reaction is completed, the reaction solution is poured into a large amount of ice water, extracted with ethyl acetate (600ml×3), combined with ethyl acetate, Dry, filter, and concentrate to obtain 7.9 g of yellow liquid, with a yield of 61%.
[0053]The preparation of 2.3-bromo-4-formyl butyric acid ethyl ester (V)
[0054] Compound (IV) (7.9 g, 0.06 mol) and bromotrimethylsilane (11 g, 0.072 mol) were added to the reaction flask, the temperature was lowered to 0° C., and triethylamine (6 g, 0.06 mol) was added dropwise. After dropping, the temperature was raised to reflux, and after 4 hours it was cooled to room temperature, 200ml of chloroform was added, and a soluti...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com