Aptamer and new use of derivative thereof
A nucleic acid aptamer and derivative technology, which is applied in the direction of non-active ingredient medical preparations, drug combinations, anti-infectives, etc., can solve difficult chemical modification and transformation, poor stability of antibody conjugates, easy to cause immune reactions, etc. problem, to achieve the effect of low cost, good stability, no immune activity and toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Example 1, Screening, Optimization and Transformation of Aptamer I and Aptamer II
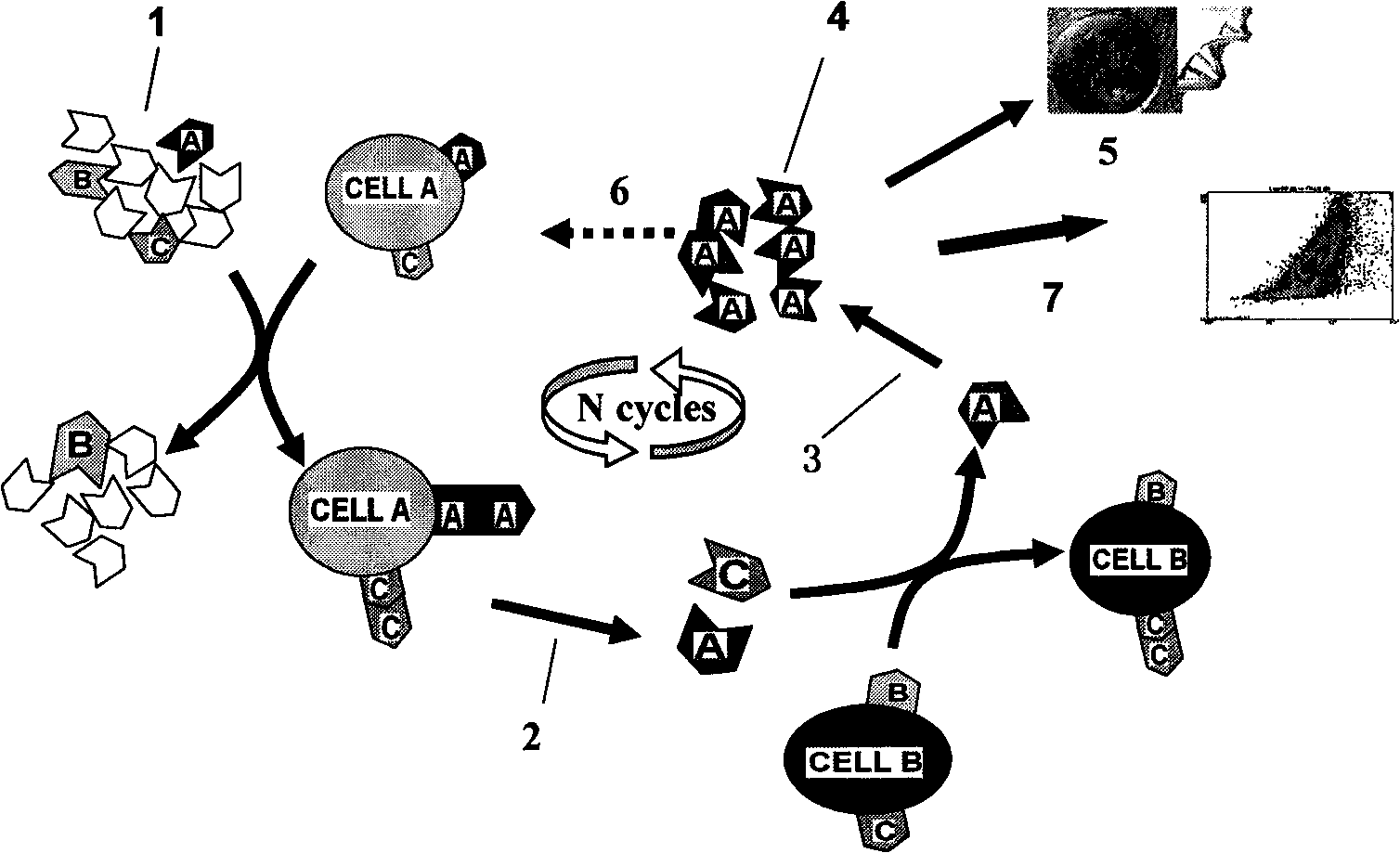
[0047] Screening process such as figure 1 As shown (cell A is the target cell, cell B is the counter-screened cell, 1 represents the random DNA library, 2 represents the elution step, 3 represents the PCR step, 4 represents the enriched library, 5 represents cloning and sequencing, 6 represents the entry into the next One round of screening, 7 represents the flow cytometric analysis step), as follows:
[0048] 1. Screening, optimization and transformation of CCRF-CEM nucleic acid aptamers in leukemia cells
[0049] (1) Screening of nucleic acid aptamers
[0050] 1. Design and synthesis of random nucleic acid library
[0051] Design and synthesize a random nucleic acid sequence library containing 18 nucleotides at both ends and 52 nucleotides in the middle as follows: 5'-ATA CCA GCT TAT TCA ATT-52-nt-AGA TAG TAA GTG CAA TCT-3'.
[0052] 2. Screening of nucleic acid aptamers
[0053] ...
Embodiment 2
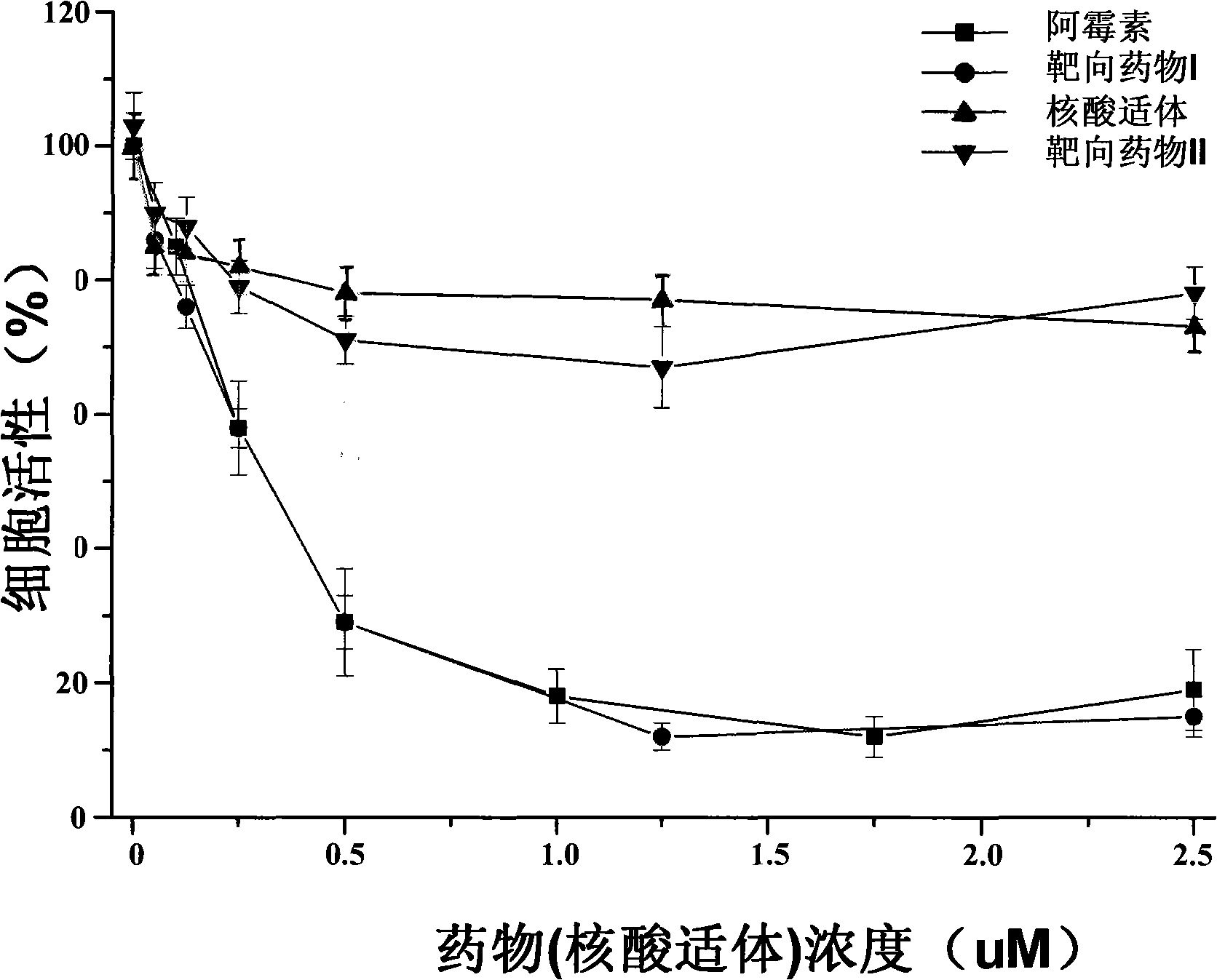
[0104] Embodiment 2, preparation and application of targeted drug
[0105] 1. Preparation of Targeted Drug I
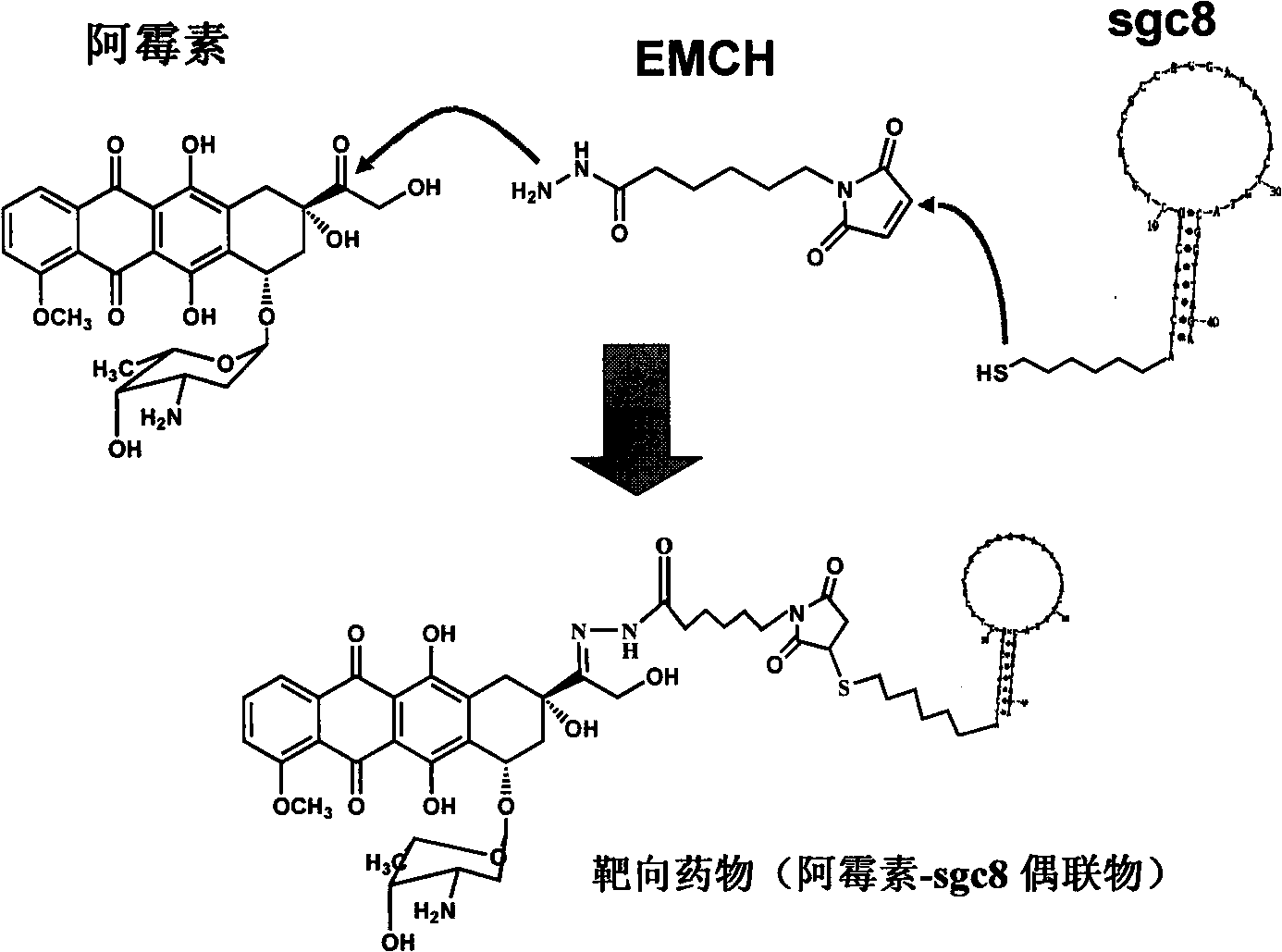
[0106] The preparation process is as figure 2 shown.
[0107] 1. Preparation of doxorubicin 6-maleimide caproyl hydrazone
[0108] Take doxorubicin hydrochloride (5 mg, 8.62 μmol) and EMCH (N-e-(Maleimidocaproic acid) hydrazide, N-e-maleimidocaproic acid hydrazide) (Pierce Biotechnology) (10 mg, 44.4 μmol) dissolved in 4 mL methanol, add 3 μL Trifluoroacetic acid reacted at room temperature in the dark for 24 hours; then concentrated under reduced pressure at room temperature to 0.25 mL, added 2.5 mL of acetonitrile and placed at 4°C for 48 hours for recrystallization; washed the crystals with a methanol-acetonitrile (1:10) mixture, and dried in vacuo to obtain Doxorubicin 6-maleimide caproylhydrazone (2.9 mg, 3.85 μmol).
[0109] 2. Coupling of nucleic acid aptamers and drugs
[0110] Take 300nmol nucleic acid aptamer A modified by the disulfide bond at the 5' ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com