Method and applications thereof for recovering MnO* from anode material of wasted lithium manganate battery
A battery cathode, old lithium manganate technology, applied in the field of waste material recycling, can solve problems such as high cost, complex recycling process, complex electrode material composition, etc., to save energy, avoid secondary pollution, and avoid high-temperature calcination processes. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Weigh 50g of the positive electrode sheet of the spent lithium manganese oxide battery, and dissolve the aluminum foil of the positive electrode current collector with 100mL of 1-10mol / L NaOH solution. Among them, aluminum is represented by NaAlO 2 The form enters the solution to be recovered, and the positive electrode active material falls off naturally and becomes alkali leaching residue. Wash the alkali leaching residue repeatedly with distilled water until the washing liquid is neutral, and use 100mL N-methylpyrrolidone (NMP) to wash the above-mentioned alkali leaching residue 3 to 4 times to remove the binder polyvinylidene fluoride (PVDF ). After the above pretreatment, the positive electrode active material contains LiMn 2 o 4 Waste cathode materials and activated carbon. The active substance washed with NMP is repeatedly washed with distilled water for 4-5 times, dried and ground for later use.
Embodiment 2
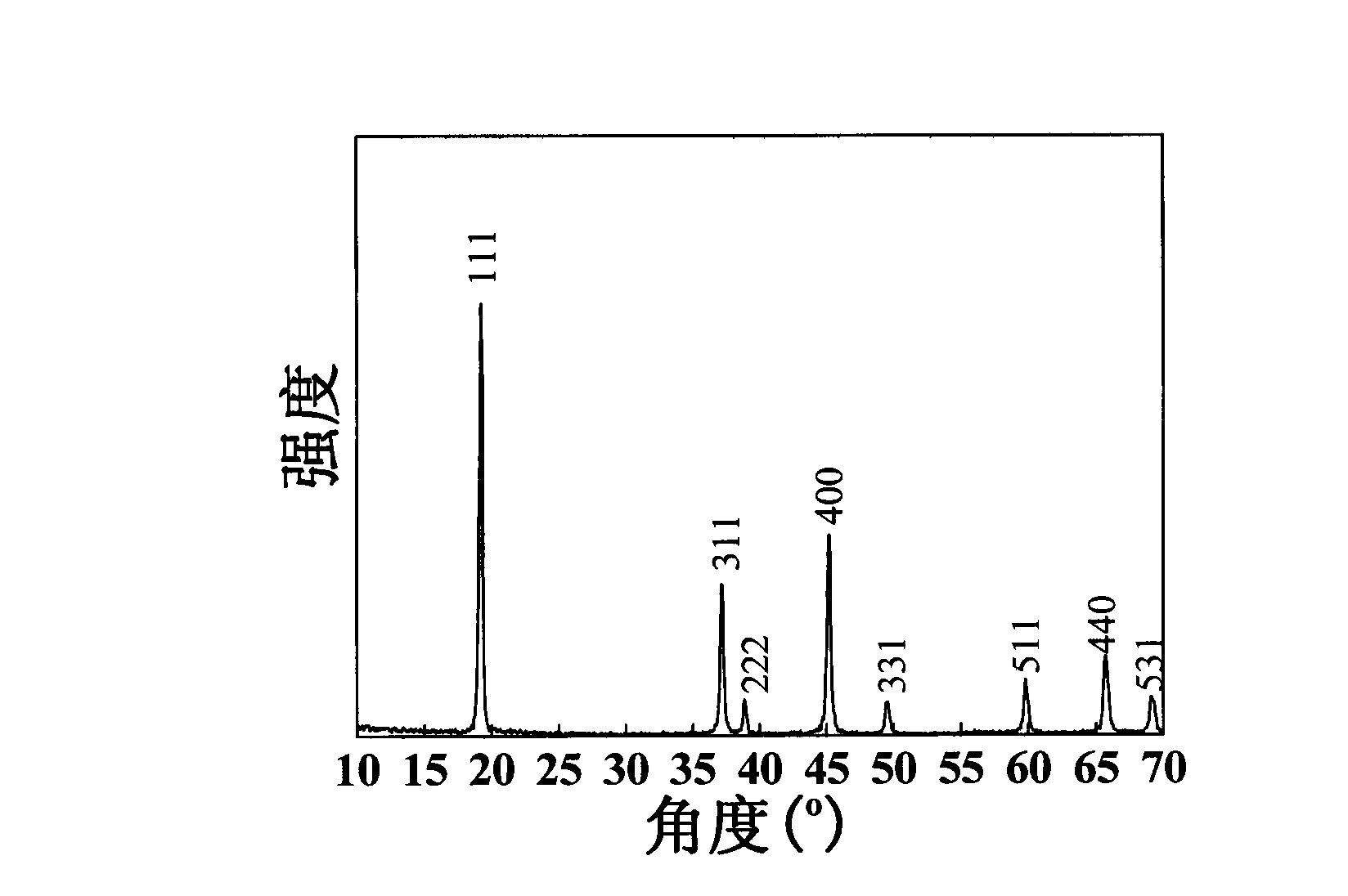
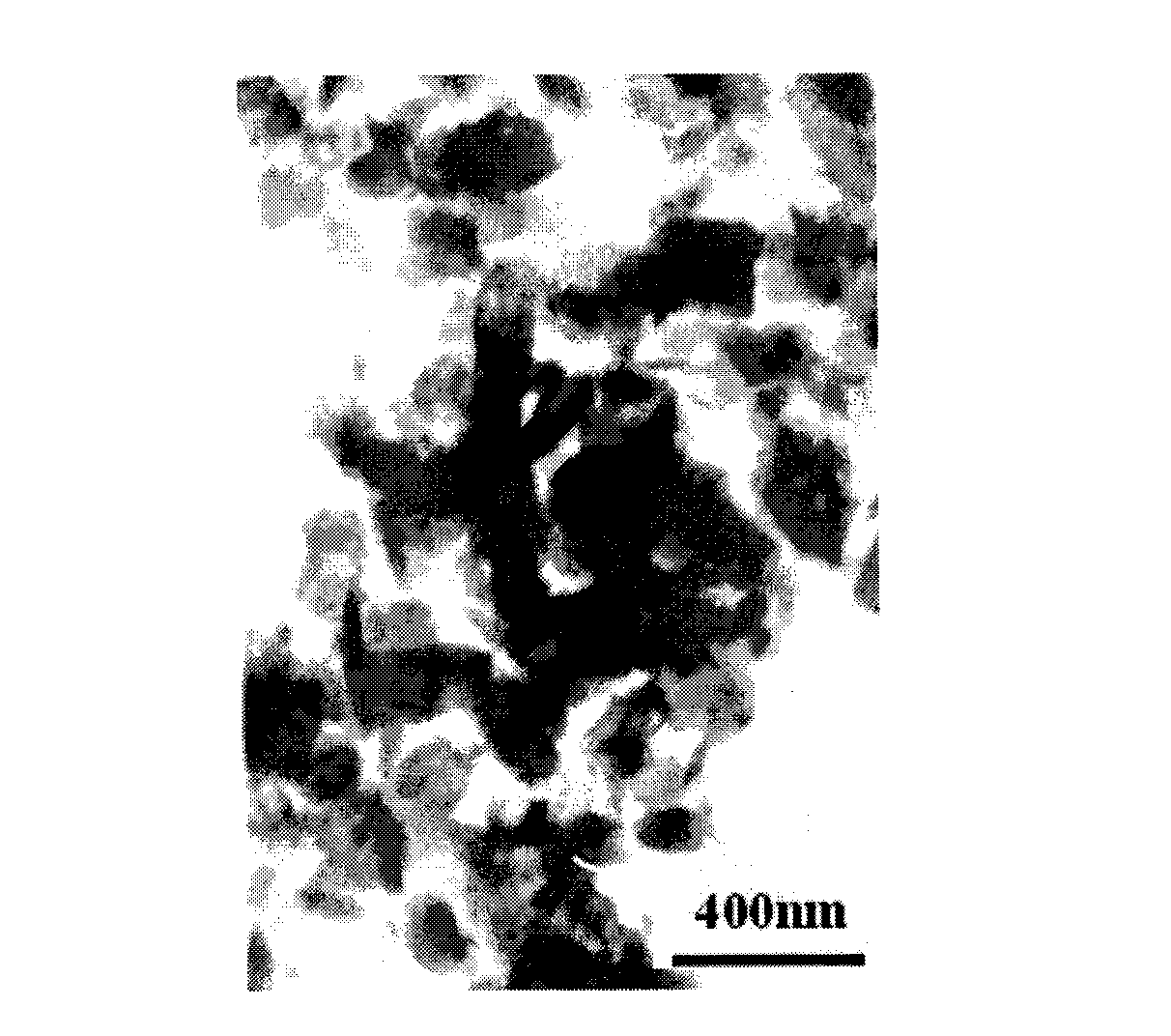
[0042] Preparation of MnO from the positive electrode active material recovered after the above pretreatment 2 . Weigh 9.77g of positive electrode active material, place in 100mL of 0.25-5mol / L H 2 SO 4 In the solution, under the action of magnetic stirring, react at room temperature (25°C) and normal pressure for 1-10 hours, and the suspension appears dark red. The acid leaching residue was separated by suction filtration from the suspension, washed with distilled water, and dried at 100°C for use. The XRD pattern of the material obtained by atmospheric acid leaching is shown in figure 1 After analysis, the product has a similar crystal structure to the lithium manganate cathode material in the original waste battery, which is the cubic λ-MnO 2 . figure 2 It is a TEM photo of the material, it can be seen that after acid leaching at atmospheric pressure, the prepared λ-MnO 2 The particle size is about 200-300nm.
Embodiment 3
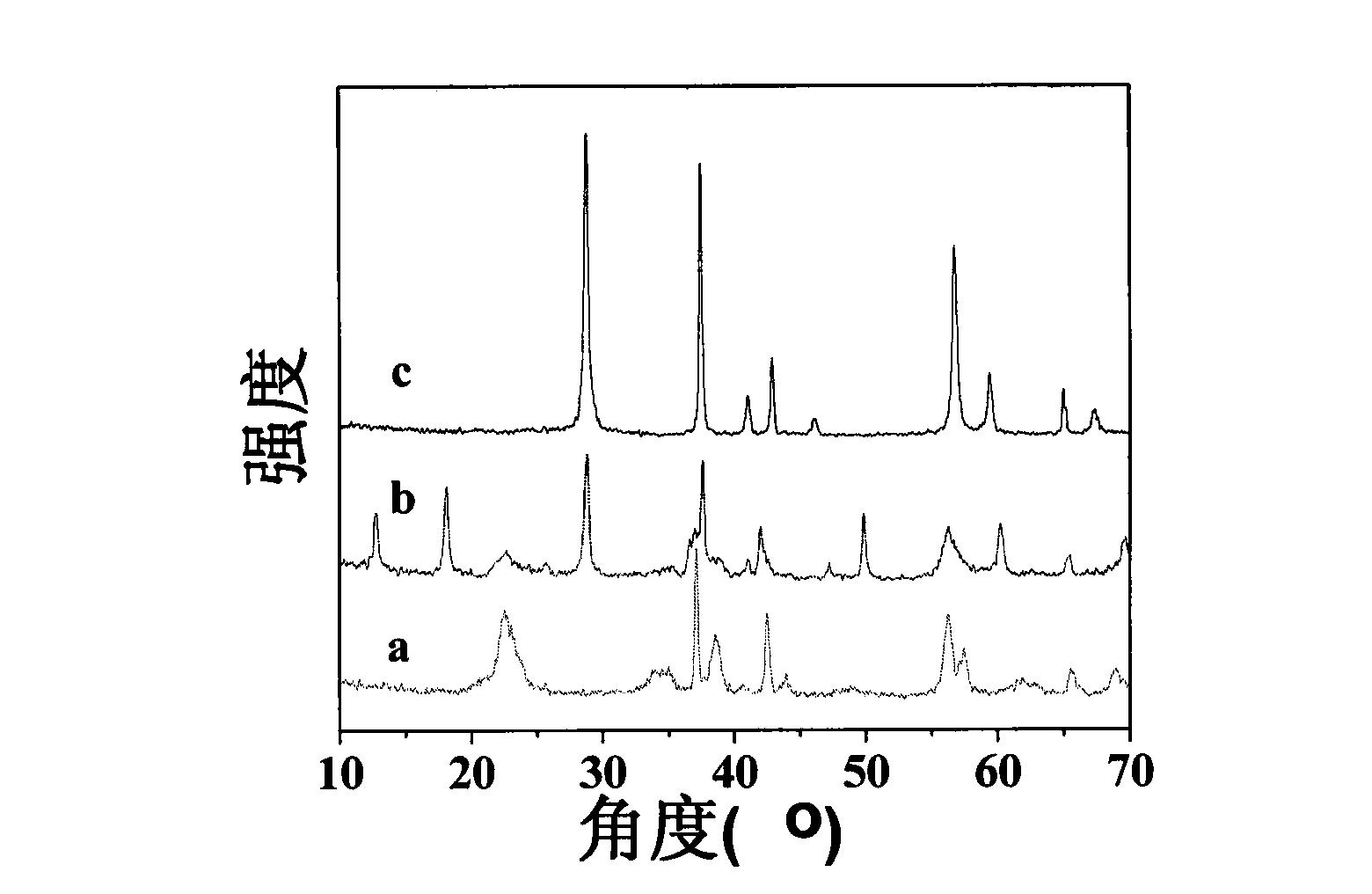
[0044] Add 9.77g of the positive electrode active material recovered after pretreatment to 40mL of 1.5-10mol / L H 2 SO 4 In the solution, stir it with a glass rod to make it fully mixed, then place it in a high-pressure reactor, and carry out a hydrothermal reaction at 100-200°C and a pressure of 0.5-10Mpa for 1-60h. The obtained precipitate was separated by centrifugation, washed with water until neutral, and dried at 100°C for future use. image 3 The XRD patterns of each product were obtained for hydrothermal acid leaching at different times. It can be seen from the figure that when the acid leaching time is 1.5-3h, the product is orthorhombic γ-MnO 2 ( image 3 a); With the prolongation of acid leaching time, to 6-24h, the product is tetragonal α-MnO 2 ( image 3 b); as the acid leaching time is further extended to 30-60h, the product is tetragonal β-MnO 2 ( image 3 c). Figure 4 For the prepared three crystal forms of MnO 2 TEM photographs. from Figure 4 It ca...
PUM
| Property | Measurement | Unit |
|---|---|---|
| length | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com