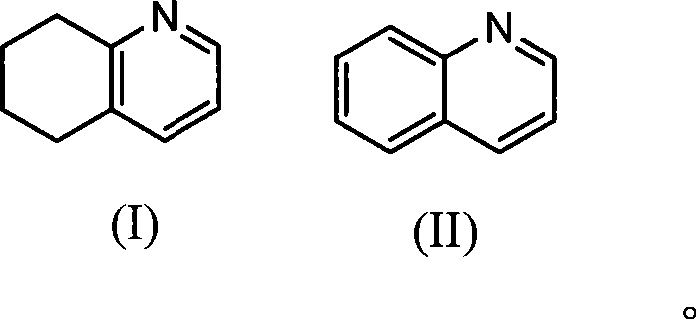
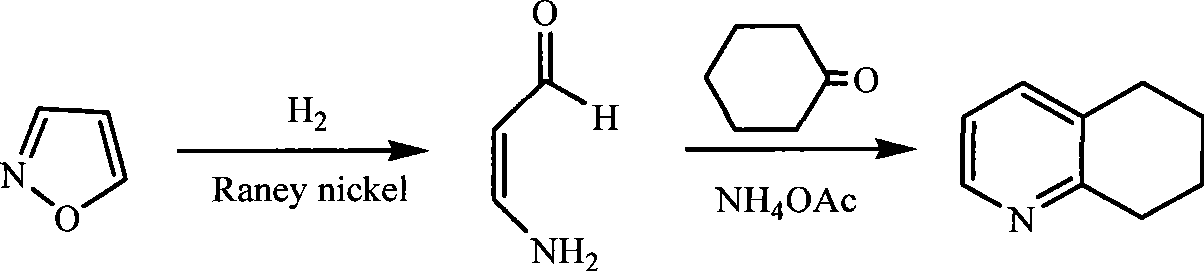
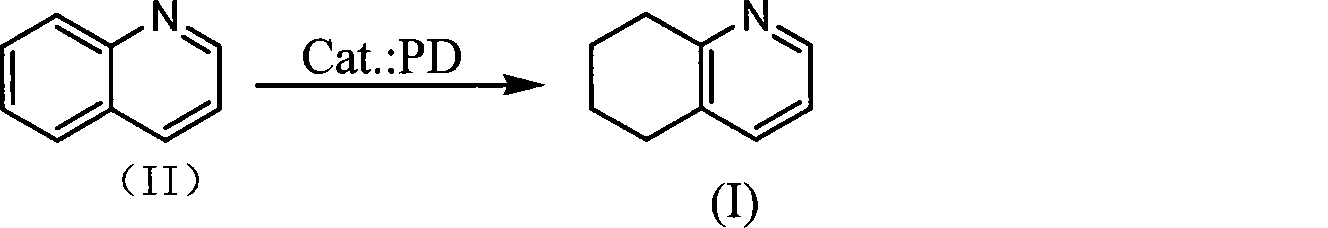
Method for synthesizing 5,6,7,8-tetrahydroquinoline
A technology of tetrahydroquinoline and synthetic method, which is applied in the field of key intermediates of cefquinidine, can solve the problems of high equipment requirements, low yield, large pollution, etc., and achieve low reaction temperature, high yield, and low material loss. The effect of energy consumption reduction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Embodiment 1: with CuCl 2 Preparation of PD catalyst
[0028] Add 20g of palladium carbon (5wt% palladium loading) purchased from Zhejiang Metallurgical Research Institute Co., Ltd. to 100mL CuCl 2 (2mol / L) aqueous solution, stir and heat to about 50°C, then add 400mL NaHCO 3 Solution (1mol / L), continued to stir for 2 hours, filtered, and the filter cake was washed with 2×20mL water, and vacuum-dried at 130°C to obtain 32.4g of catalyst, which was designated as PD-1.
Embodiment 2
[0029] Embodiment 2: with ZnCl 2 Preparation of PD catalyst
[0030] Add 10g of palladium carbon (5%) purchased from Zhejiang Metallurgical Research Institute Co., Ltd. to 50mLZnCl 2 (1mol / L) aqueous solution, under stirring, add 200mL NaHCO at about 10°C 3 Solution (0.5mol / L), continued to stir for 3 hours, filtered, and the filter cake was washed with 2×20mL water, and vacuum-dried at 120°C to obtain 13.2g of catalyst, which was designated as PD-2.
Embodiment 3
[0031] Embodiment 3: with FeCl 2 Preparation of PD catalyst
[0032] Add 10g of palladium carbon (5%) purchased from Zhejiang Metallurgical Research Institute Co., Ltd. to 40mLFeCl 2 (5mol / L) aqueous solution, stir and heat to about 30°C, add 210mL NaHCO 3 Solution (1.9mol / L), continued to stir for 2 hours, filtered, and the filter cake was washed with 2×20mL water, and vacuum-dried at 100°C to obtain 22.5g of catalyst, which was designated as PD-3.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com