Method for preparing triclabendazole
A technology of triclabendazole and dichloroaniline, which is applied in the field of preparation of triclabendazole, can solve the problems of high price, unsafety, and high risk, and achieve industrial production, avoid high-pressure reaction, and be environmentally friendly Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
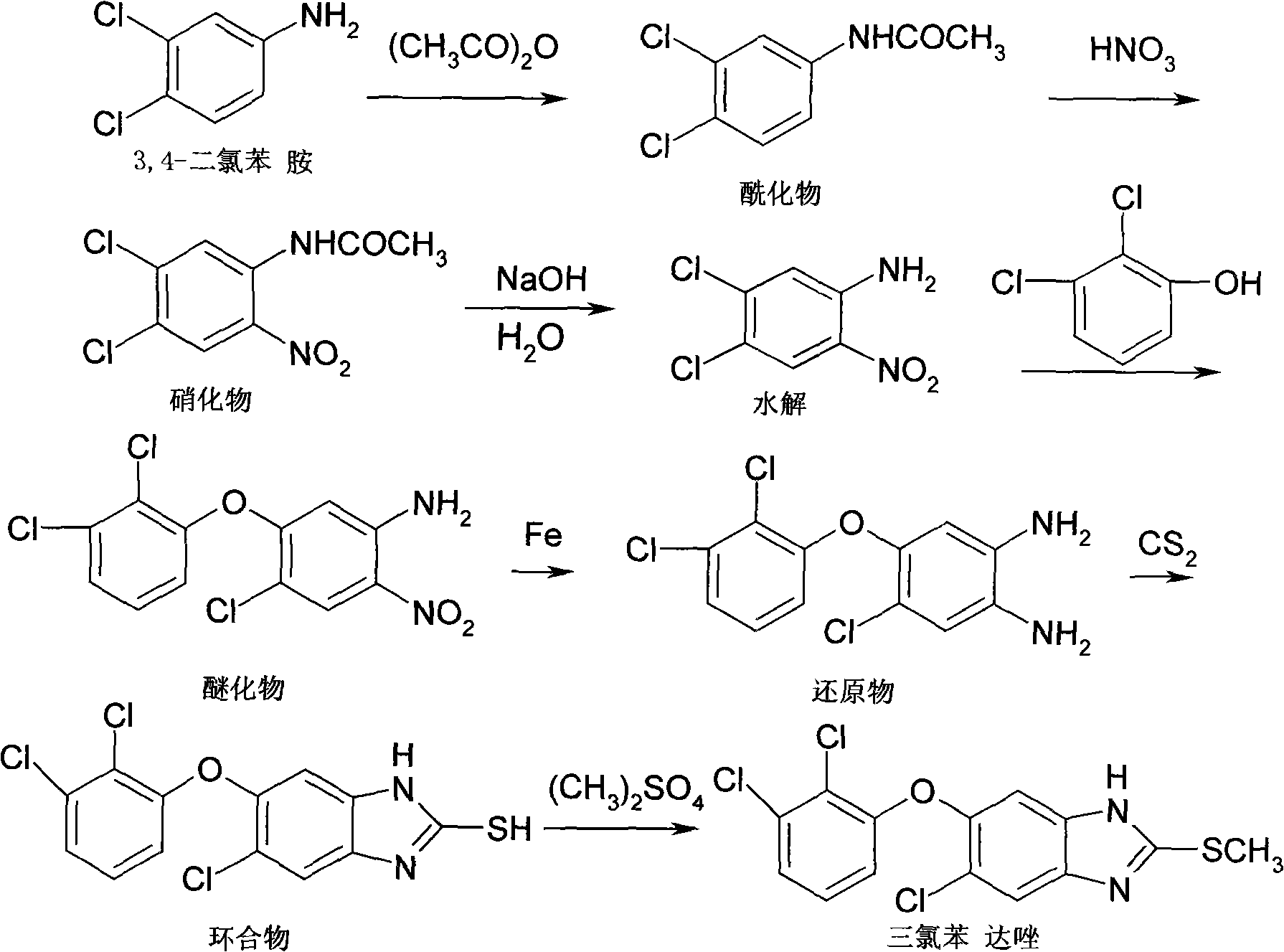
[0016] Steps:
[0017] 1. Preparation of acylate {3,4-dichloroacetanilide (3)}:
[0018] Put 10g of 3,4-dichloroaniline into the reaction flask at a temperature of 80-145°C, add 100g of acetic acid and stir to dissolve, heat up to reflux, add 15g of acetyl chloride dropwise, finish adding, reflux for 5 hours, exhaust the hydrogen chloride gas, Then, 200 ml of water was added, followed by suction filtration to obtain 11 g of acylate.
[0019] 2. Preparation of nitrate {4,5-dichloro-2-nitroacetanilide (4)}:
[0020] Put 20-150g of sulfuric acid and 10g of acylate into the reaction bottle, and add 18g of nitrating agent dropwise at the temperature of -10-40°C. Recrystallized to obtain 9g of refined product. Above nitrating agent can be nitric acid, also can be the mixture of nitric acid and acetic acid, can also be the mixture of nitric acid and sulfuric acid, or potassium nitrate.
[0021] 3. Preparation of hydrolyzate {4,5-dichloro-2-nitroaniline (5)}:
[0022] Under the t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com