Disperse red dye
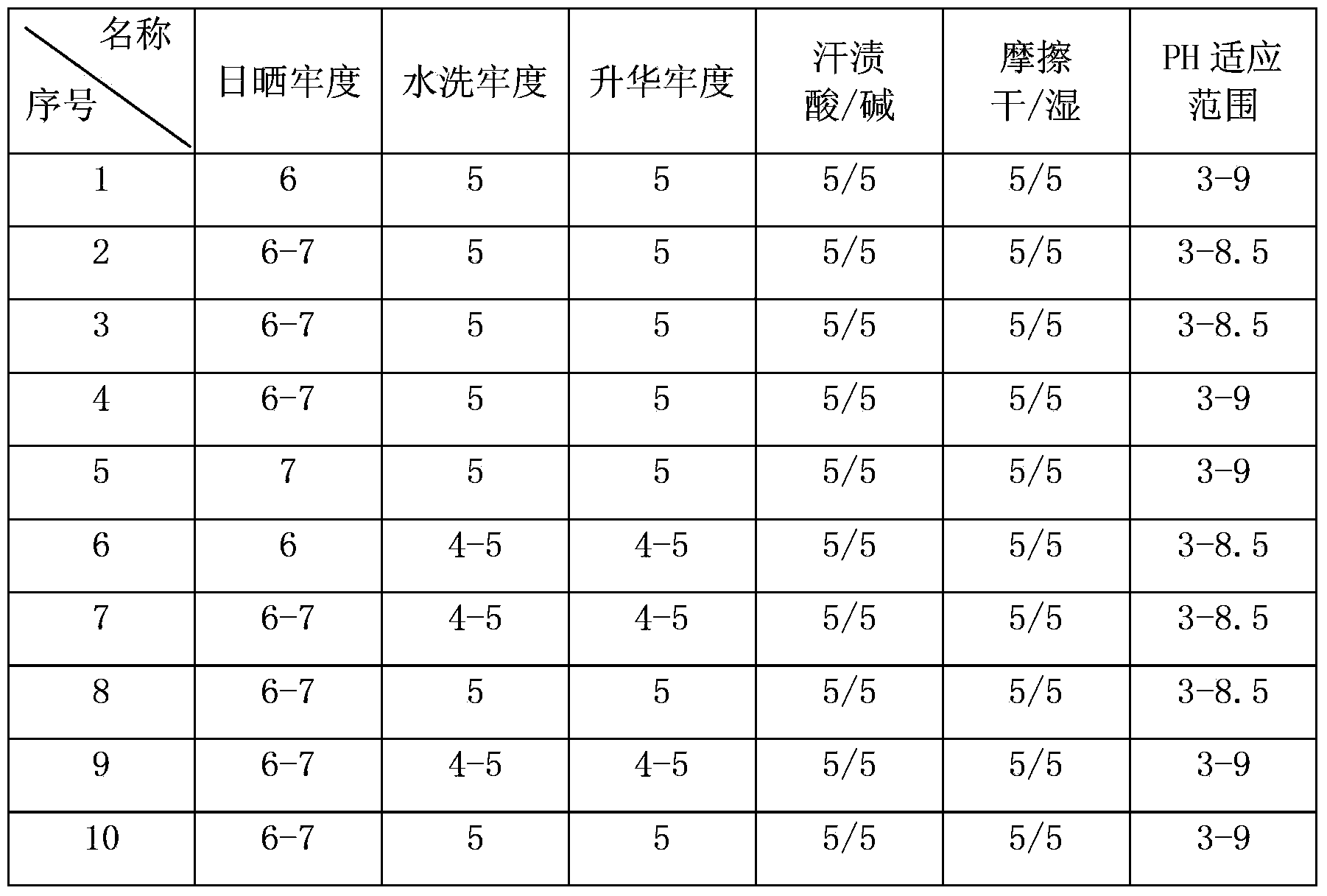
A disperse dye and monoazo technology, applied in the field of red disperse dyes, can solve the problems of small pH adaptability range, low product strength, easy fading, etc., and achieve strong washing fastness, strong light fastness, and wide pH adaptability range Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
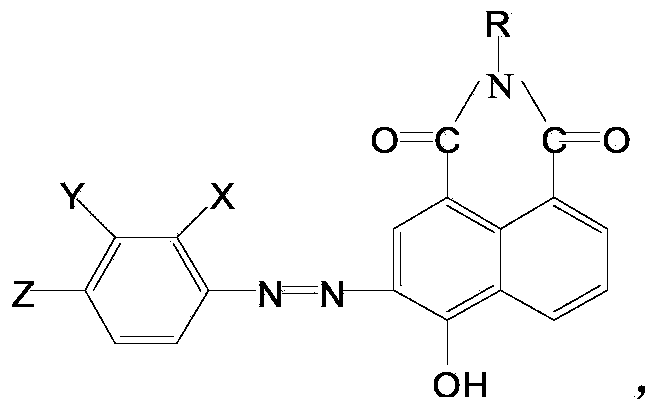
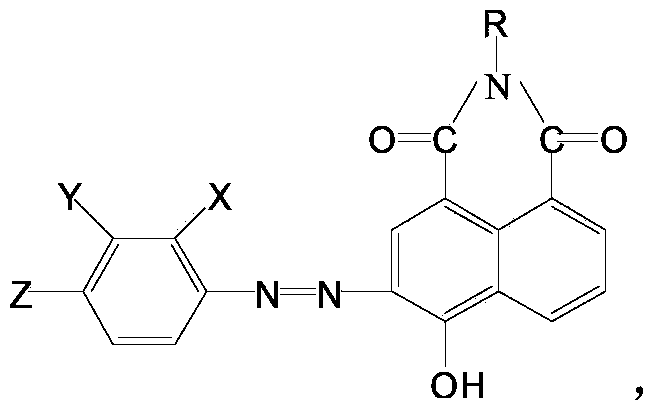
[0019] Put 10ml of 30% hydrochloric acid into the flask, add 20ml and 4g of p-nitroaniline, heat up to 95°C, keep warm for 1 hour, then cool down to 30°C, add 40ml of water, then add 30g of ice, keep the bottle in an ice bath The internal temperature is 0-5 degrees, add 2.5g sodium nitrite 10% solution, keep the reaction for 2 hours, after the reaction is completed, after 2 hours at 0-5 degrees, add the above mixture by 20ml ice water, 4gNaOH and 4-Hydroxy-N-methyl-1,8-naphthoimide (abbreviated as red intermediate, see the following formula for structure).
[0020] R=CH3, CH2CH3 or CH2CH2CH2OCH3.
[0021] After continuing to stir for 2 hours, add 5ml of hydrochloric acid dropwise, continue to stir for 1 hour, heat up to 80 degrees, and keep it warm for 2 hours. The obtained dye is suction filtered, washed with clear water and dried. The reaction product is:
[0022] R=CH3, CH2CH3 or CH2CH2CH2OCH3, max=520nm. After the dyestuff is processed, it becomes bright yellow and l...
Embodiment 2~10
[0024] Replace the p-nitroaniline in Example 1 with o-chloro-p-nitroaniline, red base 3GL, o-methoxy p-nitroaniline, phenyl m-aminobenzenesulfonate, aniline, 2,5-dichloroaniline , 3,4-dichloroaniline, o-nitroaniline, m-nitroaniline, keeping other conditions constant, the reaction products are respectively:
[0025] Coupling of o-chloro-p-nitroaniline and red intermediate
[0026] R=CH3, CH2CH3 or CH2CH2CH2OCH3, into max=520nm
[0027] Coupling of red-based 3GL and red intermediates
[0028] R=CH3, CH2CH3 or CH2CH2CH2OCH3, into max=520nm
[0029] Coupling of o-methoxy-p-nitroaniline and red intermediate
[0030] R=CH3, CH2CH3 or CH2CH2CH2OCH3, into max=550nm
[0031] Coupling of phenyl m-aminobenzenesulfonate and red intermediate
[0032] R=CH3, CH2CH3 or CH2CH2CH2OCH3, into max=510nm
[0033] Coupling of aniline and red intermediates
[0034] R=CH3, CH2CH3 or CH2CH2CH2OCH3, into max=510nm
[0035] Coupling of 2,5-dichloroaniline and red intermediate
[0036]...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com