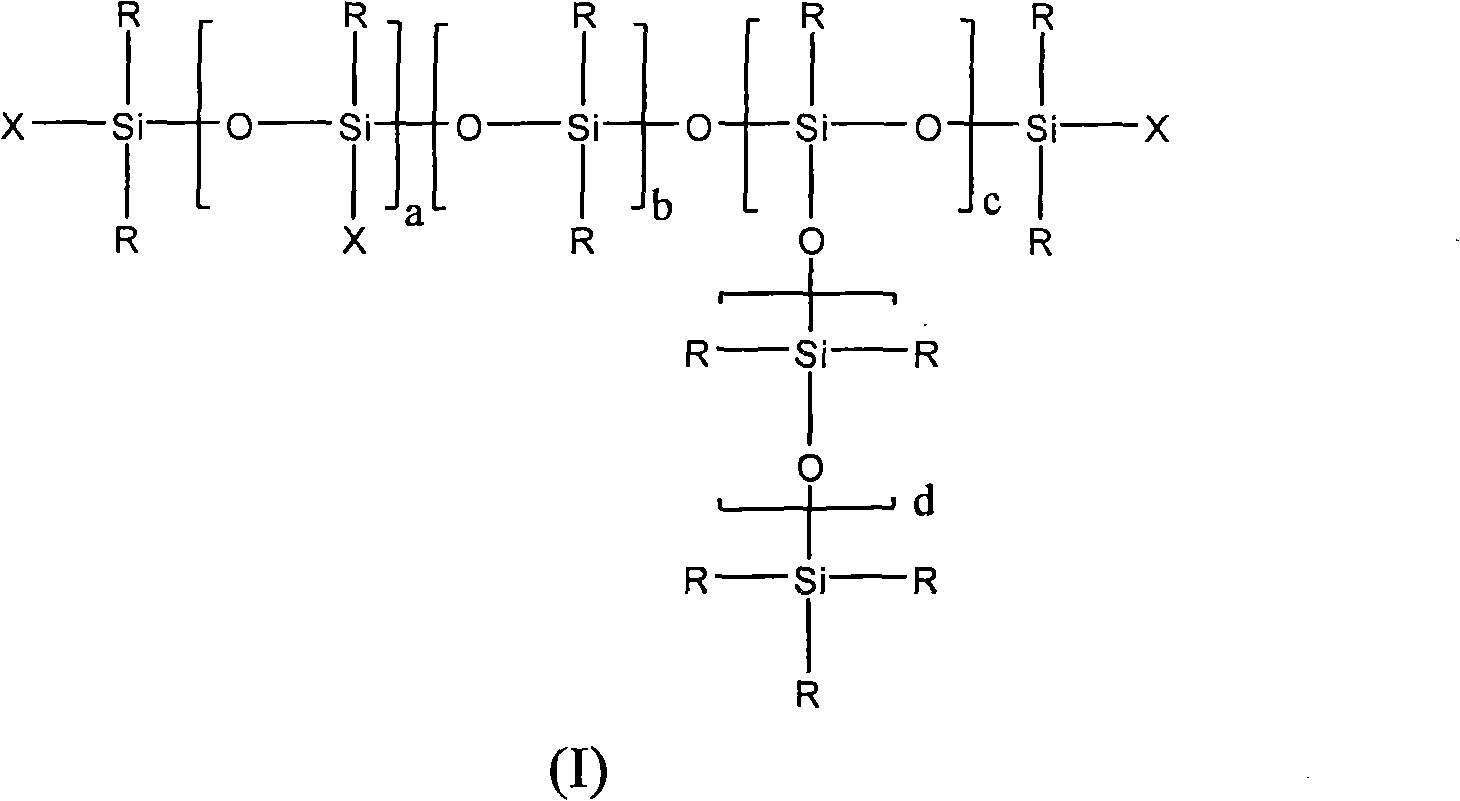
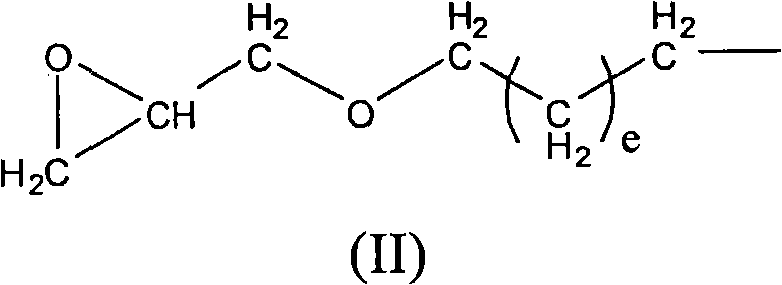
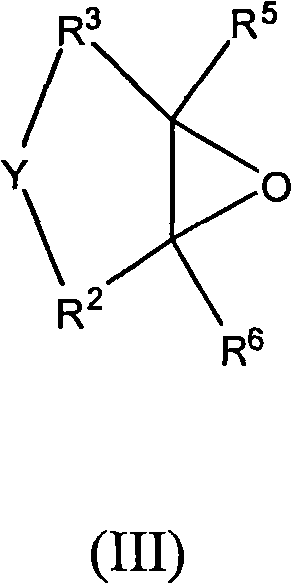
New polyether alcohols containing organosiloxane groups by using double metal cyanide and preparation method thereof
A technology of siloxane polyether and siloxane alkoxy, which is applied in the field of new polyether alcohols, can solve the problems of reducing the concentration of surface-active siloxane polyether and damaging the performance of target products, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0111] Under nitrogen, 200.0 g of polypropylene glycol monobutyl ether (average molar mass 750 g / mol) and 0.017 g of zinc hexacyanocobaltate DMC catalyst were initially charged to a 3-liter autoclave and heated to 130° C. with stirring. The reactor was pumped down to an internal pressure of 30 mbar, so that any volatile constituents present were removed by distillation. To activate the DMC catalyst, a 50.0 g portion of propylene oxide was supplied. After 20min and reaction initiation (reactor internal pressure drop), 100.0g epoxysiloxane and 368.0g propylene oxide based on heptamethyltrisiloxane and allyl glycidyl ether were continuously Cooling, while metering in at 130° C., the internal pressure of the reactor was a maximum of 1.2 bar abs. A post-reaction was carried out at 130-150°C for 180 minutes, followed by a degassing step. In this step, volatile components such as residual propylene oxide are distilled off under reduced pressure. The colorless, low-viscosity and tr...
Embodiment 2
[0114] Under nitrogen, 200.0 g of polypropylene glycol monobutyl ether (average molar mass 750 g / mol) and 0.015 g of zinc hexacyanocobaltate DMC catalyst were initially charged to a 3-liter autoclave and heated to 130° C. with stirring. The reactor was pumped down to an internal pressure of 30 mbar, so that any volatile constituents present were removed by distillation. To activate the DMC catalyst, a 50.0 g portion of propylene oxide was supplied. After 20min and reaction initiation (reactor internal pressure drops), the 100.0g epoxysiloxane based on heptamethyltrisiloxane and allyl glycidyl ether and 463.0g 1,2-epoxybutane The homogeneous mixture, cooled continuously within 35 min, is metered in at 130° C., the internal pressure of the reactor being a maximum of 0.8 bar abs. A post-reaction was carried out at 135-150°C for 180 minutes, followed by a degassing step. In this step, volatile components such as residual 1,2-butylene oxide are distilled off under reduced pressur...
Embodiment 3
[0117] Under nitrogen, 200.0 g of polypropylene glycol monobutyl ether (average molar mass 2200 g / mol) and 0.015 g of zinc hexacyanocobaltate DMC catalyst were initially charged to a 3-liter autoclave and heated to 130° C. with stirring. The reactor was pumped down to an internal pressure of 30 mbar, so that any volatile constituents present were removed by distillation. To activate the DMC catalyst, a portion of 58.0 g of propylene oxide was supplied. After 15min and reaction initiation (reactor internal pressure drops), 6 parts of each part of 16.0g epoxysiloxane and 26.0g propylene oxide based on heptamethyltrisiloxane and allyl glycidyl ether were Cooling within 5 hours, metered in alternately at 125° C., the internal pressure of the reactor is a maximum of 1.0 bar abs. A post-reaction was performed at 125-150°C for 150 minutes, followed by a degassing step where volatile components such as residual propylene oxide were distilled off under reduced pressure. The resulting...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Average molar mass | aaaaa | aaaaa |
| Average molar mass | aaaaa | aaaaa |
| Average molar mass | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com