Ferriferous oxide/tin oxide core-shell nanometer rod absorbing high-frequency electromagnetic wave and preparing method thereof
A technology of triiron tetroxide and nanorods, applied in the field of nanomaterials, can solve the problems of the width of the absorption frequency and the low absorption intensity, and achieve the effect of simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] (1) 40 milliliters of 0.5mol / L FeCl 3 The solution was placed in a 50ml stainless steel sealed autoclave. In an oven at 120°C for 12 hours. After the autoclave was naturally cooled to room temperature, the precipitate in the autoclave was washed with water and ethanol several times. After drying at 80°C, β-FeOOH nanorods were obtained, and then annealed at 500°C for 2.5 hours to obtain α-FeOOH nanorods 2 o 3 Nano stave;
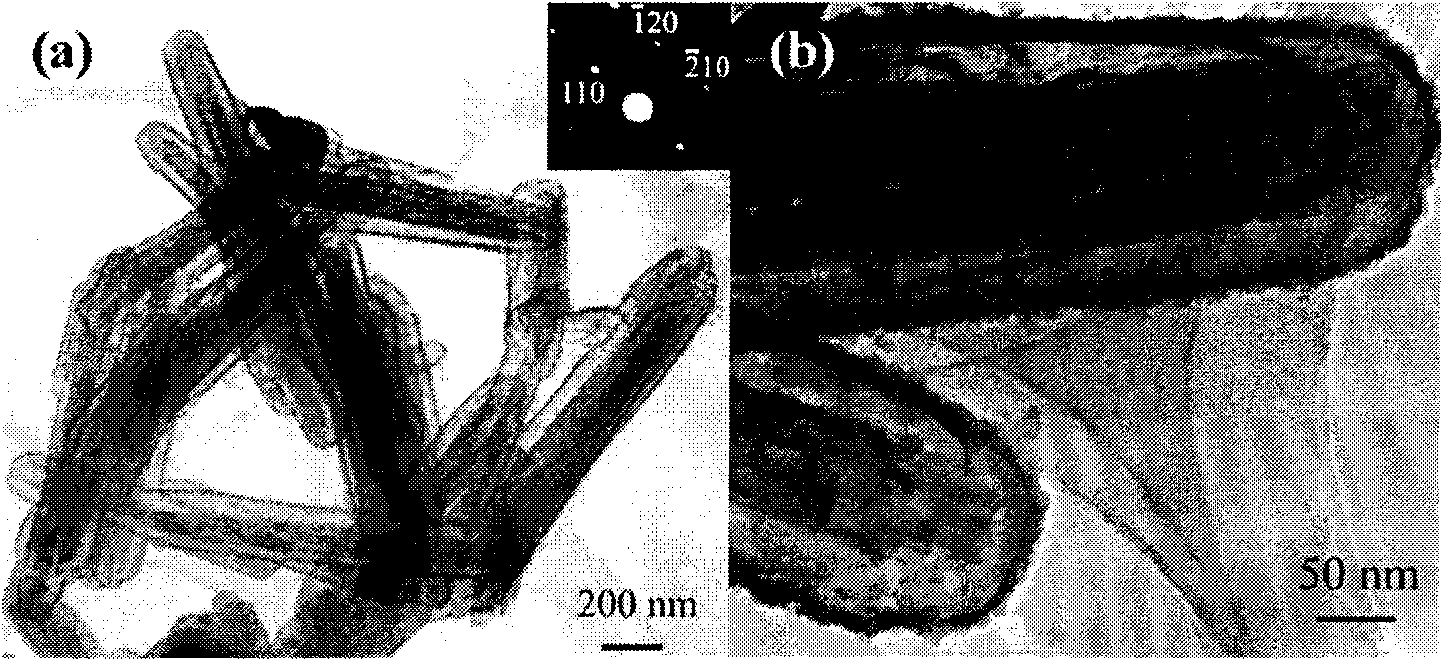
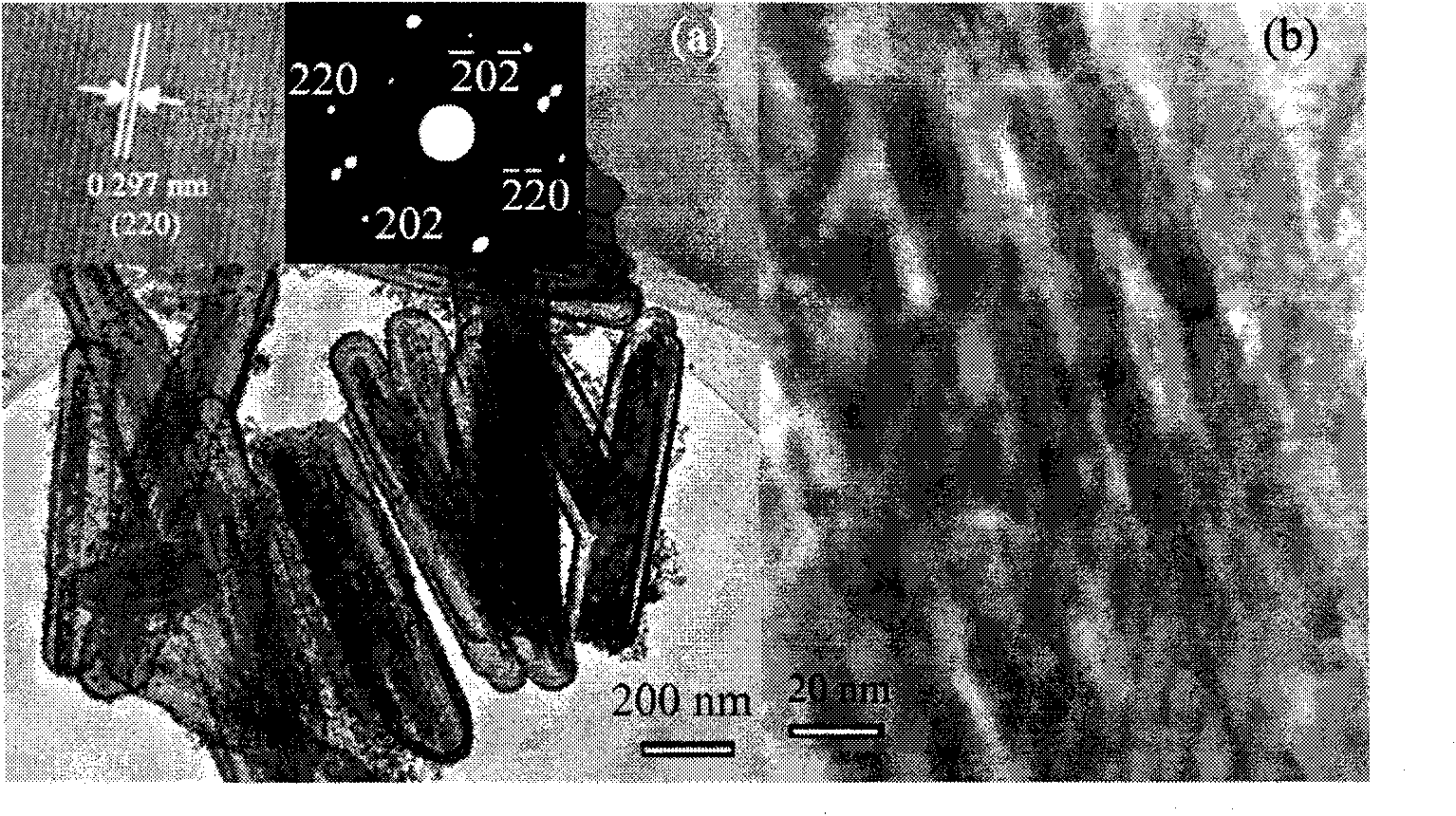
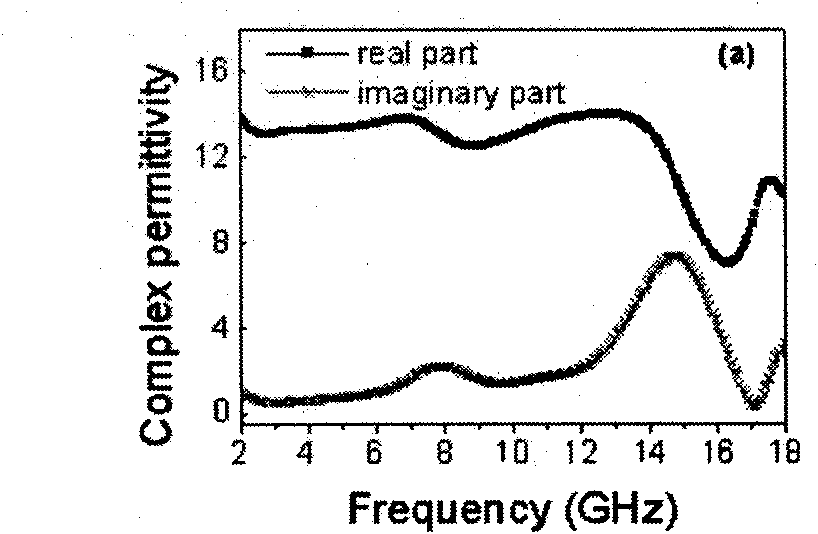
[0021] (2) 0.08g α-Fe 2 o 3 The nanorods were ultrasonically dispersed into 32ml of water-ethanol solution, and then 0.75g of urea and 0.115g of potassium stannate (K 2 SnO 3 .3H 2 O, 95%). After stirring, the above solution was placed in a 50 ml stainless steel sealed autoclave. In an oven at 170°C for 36 hours. After the autoclave was naturally cooled to room temperature, the precipitate in the autoclave was washed with water and ethanol several times. α-Fe was obtained after drying at 80°C 2 o 3 / SnO 2 Core-shell nanorods, whose morph...
Embodiment 2
[0025] (1) 40 milliliters of 1.0mol / L FeCl 3 The solution was placed in a 50ml stainless steel sealed autoclave. In an oven at 100°C for 12 hours. After the autoclave was naturally cooled to room temperature, the precipitate in the autoclave was washed with water and ethanol several times. After drying at 80°C, β-FeOOH nanorods were obtained, and then annealed at 500°C for 2.5 hours to obtain α-FeOOH nanorods 2 o3 Nano stave;
[0026] (2) 0.08g α-Fe 2 o 3 The nanorods were ultrasonically dispersed into 32ml of water-ethanol solution, and then 0.75g of urea and 0.115g of potassium stannate (K 2 SnO 3 .3H 2 O, 95%). After stirring, the above solution was placed in a 50 ml stainless steel sealed autoclave. In an oven at 170°C for 36 hours. After the autoclave was naturally cooled to room temperature, the precipitate in the autoclave was washed with water and ethanol several times. α-Fe was obtained after drying at 80°C 2 o 3 / SnO 2 Core-shell nanorods;
[0027] (3)...
Embodiment 3
[0029] (1) 40 milliliters of 0.25mol / L FeCl 3 The solution was placed in a 50ml stainless steel sealed autoclave. In an oven at 110°C for 12 hours. After the autoclave was naturally cooled to room temperature, the precipitate in the autoclave was washed with water and ethanol several times. After drying at 80°C, β-FeOOH nanorods were obtained, and then annealed at 500°C for 2.5 hours to obtain α-FeOOH nanorods 2 o 3 Nano stave;
[0030] (2) 0.08g α-Fe 2 o 3 The nanorods were ultrasonically dispersed into 32ml of water-ethanol solution, and then 0.75g of urea and 0.115g of potassium stannate (K 2 SnO 3 .3H 2 O, 95%). After stirring, the above solution was placed in a 50 ml stainless steel sealed autoclave. In an oven at 170°C for 36 hours. After the autoclave was naturally cooled to room temperature, the precipitate in the autoclave was washed with water and ethanol several times. α-Fe was obtained after drying at 80°C 2 o 3 / SnO 2 Core-shell nanorods;
[0031] ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com