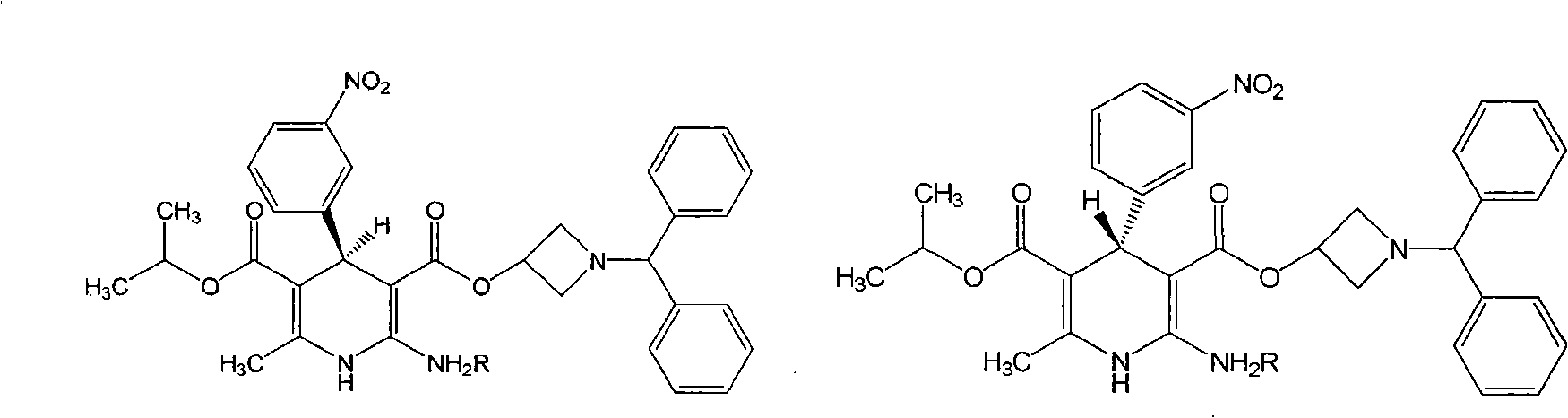
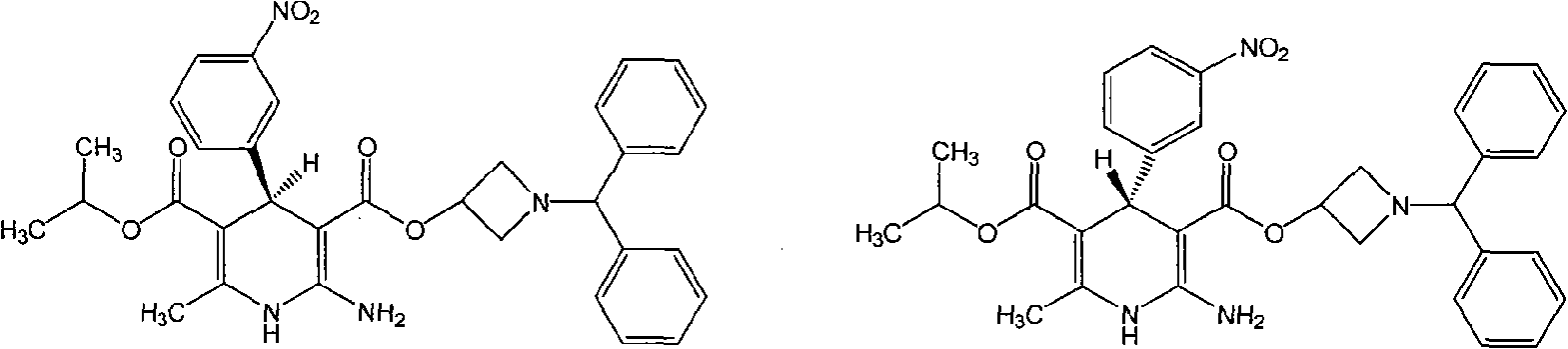
Method for preparing chiral azelnidipine and acceptable salt thereof
A technology of azeldipine and azela, which is applied in the field of preparing chiral azeldipine and can solve the problems of small processing capacity, inability to use large-scale production activities, expensive instruments and equipment, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0011] Example 1: Preparation of R-(-)-Azedipine-semi-D(-)-tartrate monosalt
[0012] Dissolve 0.29 g (0.5 mmol) of Azedipine in 5 ml of DMSO, add 0.04 g (0.5 mmol) of D-(-)-tartaric acid under heating and stirring, stir at 60°C for 1-4 hours, and filter out the solid , washed and dried with anhydrous acetone to obtain 0.11 g of R-(-)-azendipine-semi-D(-)-tartrate monosalt salt, chiral HPLC assay 99.0% d.e.
Embodiment 2
[0013] Example 2: Preparation of R-(-)-Azedipine-D(+)-camphorsulfonate monoDMSO solvate
[0014] Dissolve 0.29 g (0.5 mmol) of Azedipine in 5 ml of DMSO, add gram (mol) of D(+)-camphorsulfonic acid under stirring conditions, stir for 1-4 hours, and filter out the solid, wash and dry with n-hexane , to obtain gram R-azendipine-semi-D(+)-camphorsulfonate monoDMSO solvate, chiral HPLC assay 99.3% d.e.
Embodiment 3
[0015] Example 3: Preparation of R-(-)-Azeldipine-Semi-D(-)-Camphorsulfonate MonoDMSO Solvate
[0016] Dissolve R-(-)-azendipine-semi-D(-)-tartrate monoDMSO solvate in 20 mL of ethanol / acetone / methanol / water and add 15 mL of sodium bicarbonate / hydroxide with stirring Sodium solution, TLC detection, until the reaction is complete, ethyl acetate / n-hexane / benzene extraction 3 times, combined organic phase and vacuum distillation to obtain a solid, add methanol to dissolve, crystallize, filter to obtain R-(-)-A Zedipine, determined by chiral HPLC 99.1%, []20D=-68.2 (C=1, MeOH).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com