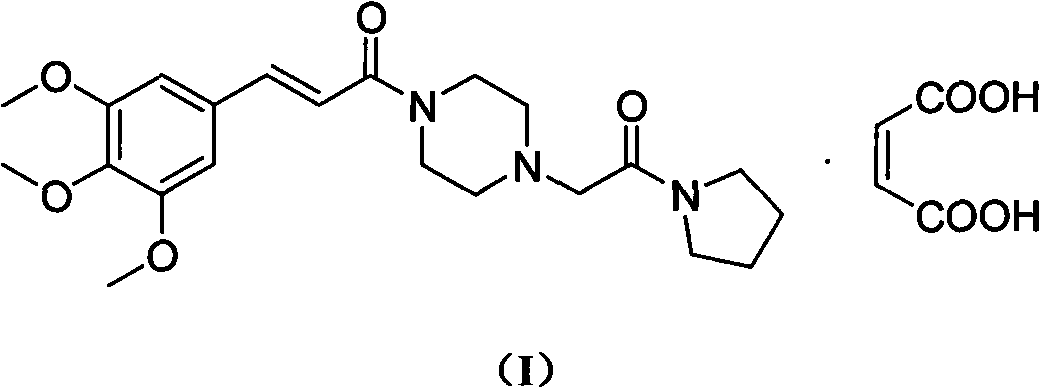
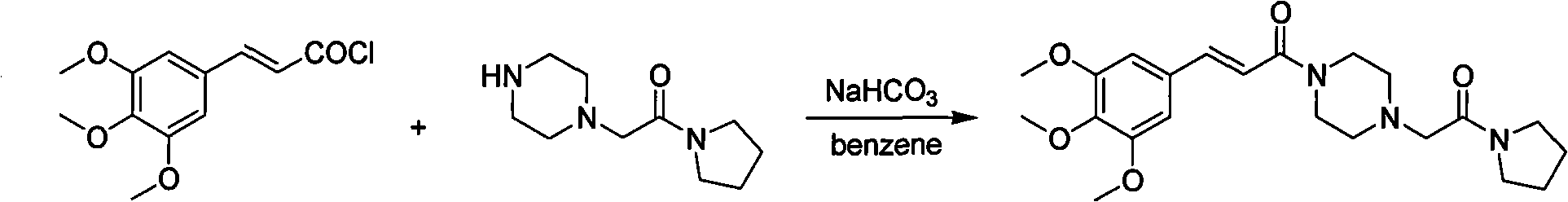
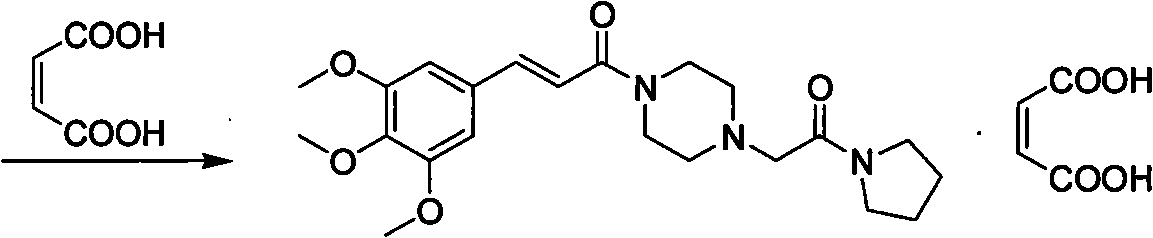
Preparation method of cinepazide maleate
A technology for the preparation of cinepazide maleate and its preparation steps, which is applied in the field of preparation of cinepazide maleate, can solve the problems of limited application, high toxicity, cumbersome handling, etc., and achieve high raw material utilization, low cost, The effect of easy process control
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0066] Synthesis of Chloroacetylpyrrolidine (II)
[0067] Chloroacetyl chloride (135g, 1.2mol) and dichloromethane (340ml) were added to a 1-liter reaction flask, cooled to -10°C in an ice-salt bath, and triethylamine (127g, 1.26mol) and pyrrolidine (85g, 1.2mol) and dichloromethane (340ml) mixed solution, control the temperature below 5 ° C, about 2 hours to complete the addition. Stir at 0-5°C for 1 hour, and at room temperature for another 1 hour. Suction filtration, the filter cake was washed with dichloromethane (510ml), and dried to obtain a brown filtrate. After the filtrate was washed with water (150ml×3), it was concentrated to obtain 158g of a brown oily substance, with a yield of 90%.
Embodiment 2
[0069] Synthesis of 1-piperazine acetylpyrrolidine (IV)
[0070] Piperazine dihydrochloride (13.28g, 0.075mol), water 70ml, anhydrous piperazine (5.60g, 0.065mol), chloroacetylpyrrolidine (7.38g, 0.05mol), use method 1, react for 7h, and then put into Anhydrous piperazine (4.31g, 0.05mol), and 40ml aqueous solution of chloroacetylpyrrolidine (7.38g, 0.05mol) were added dropwise again, and reacted for 7h, and fed in this way until 4 batches of chloroacetylpyrrolidine were added in total. 33.6 g of 1-piperazine acetylpyrrolidine was obtained, with a melting point of 65° C. and a yield of 85.12%.
Embodiment 3
[0072] Synthesis of 1-piperazine acetylpyrrolidine (IV)
[0073] Piperazine dihydrochloride (13.28g, 0.075mol), water 60ml, piperazine hexahydrate (12.62g, 0.065mol), chloroacetylpyrrolidine (7.38g, 0.05mol), use method 2, react for 9h, and then put into Piperazine hexahydrate (9.72g, 0.05mol), and a solution formed by dissolving chloroacetylpyrrolidine (7.38g, 0.05mol) in 40ml of water was added dropwise again, reacted for 8h, and fed in a circular manner in this way until a total of 6 batches of chloroacetylpyrrole were added Pyridine, 1-piperazine acetylpyrrolidine was collected to obtain 49.88g, melting point 65°C, yield 84.28%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com