Medicinal composition for inhibiting angiogenesis and application thereof
A pharmaceutical composition and angiogenesis technology, which are applied in the directions of drug combinations, medical preparations containing active ingredients, pharmaceutical formulations, etc., to achieve a good effect of inhibiting angiogenesis.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] The present invention is illustrated by the following examples, but the present invention is not limited by the following examples. Example 1 The cultivation of mycelia with the mycelium of Shanzhi Zhi (BCRC 36937)
[0039] The present invention is based on the patent certificate number I287991 of the Republic of China, the preparation method of the active substance of Cedarwood mycelia and the Cedarwood mycelium contained in the composition and the cultivation method, so as to obtain enough Cedarwood fermented liquid for freeze-drying Powder, briefly describe the cultivation method.
[0040] Mycelium strains:
[0041] It is the strain BCRC 36937 deposited in the Institute of Food Industry.
[0042] Plate culture:
[0043] The mycelium was inoculated on a plate, and cultured at 25° C. for about 2 weeks using potato dextrin medium (Potato Dextrose Agar, PDA).
[0044] Flask culture:
[0045] Scrape the hyphae on the plate and inoculate into the flask, use the ...
Embodiment 2
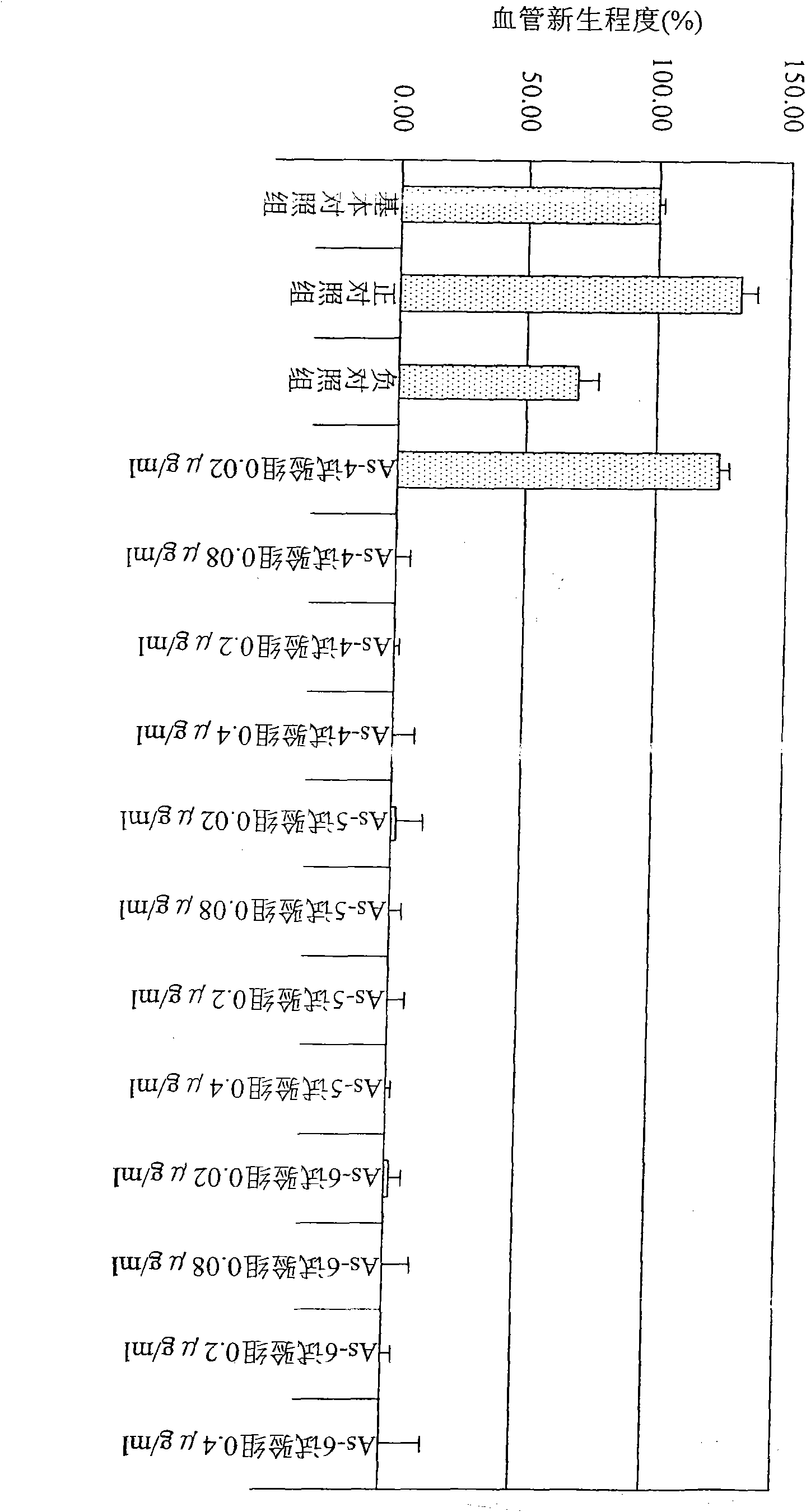
[0059] Example 2 Separation of the active components of the lyophilized powder of the fermented broth of Shanzhizhi for inhibiting angiogenesis
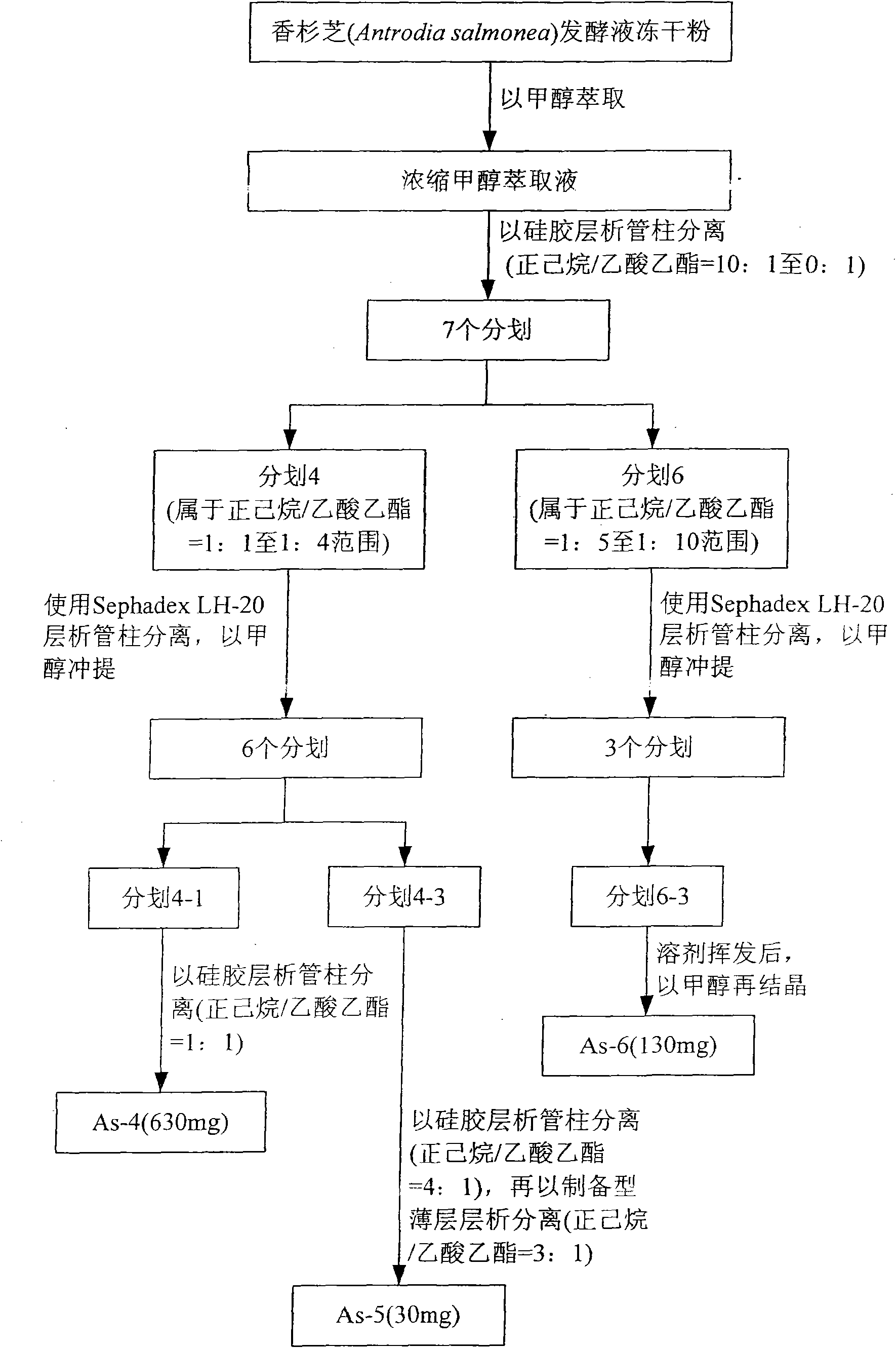
[0060] Carry out the freeze-drying of the liquid culture suspension of the mycelia of Cedarwood obtained in Example 1, including the mycelium and the clarified liquid, to obtain the lyophilized powder of the fermented liquid of C. figure 1In the purification steps shown, take 1 kg of Chinese fir fermentation broth freeze-dried powder, extract three times with four liters of methanol (MeOH) to obtain methanol extract, and concentrate to obtain methanol extract. The methanol extract was preliminarily separated on a silica gel chromatography column (60-230mesh, 5×60cm), followed by n-hexane (n-hexane) / ethyl acetate (EtOAc) at a ratio of 10:1 to 0:1 Punch and lift, get 7 divisions. It belongs to fraction 4 obtained by eluting with n-hexane / ethyl acetate (1:1 to 1:4), using Sephadex LH-20 chromatography column (3×60cm), and eluting with...
Embodiment 3
[0061] Example 3 Identification of the Active Components of the Freeze-dried Powder of the Fermented Broth of Shanzhi Zhi
[0062] The chemical structures of the three compounds obtained in Example 2 were identified, and the chemical structures of the compounds were analyzed with a mass spectrometer and a high-resolution gas chromatography-mass spectrometer.
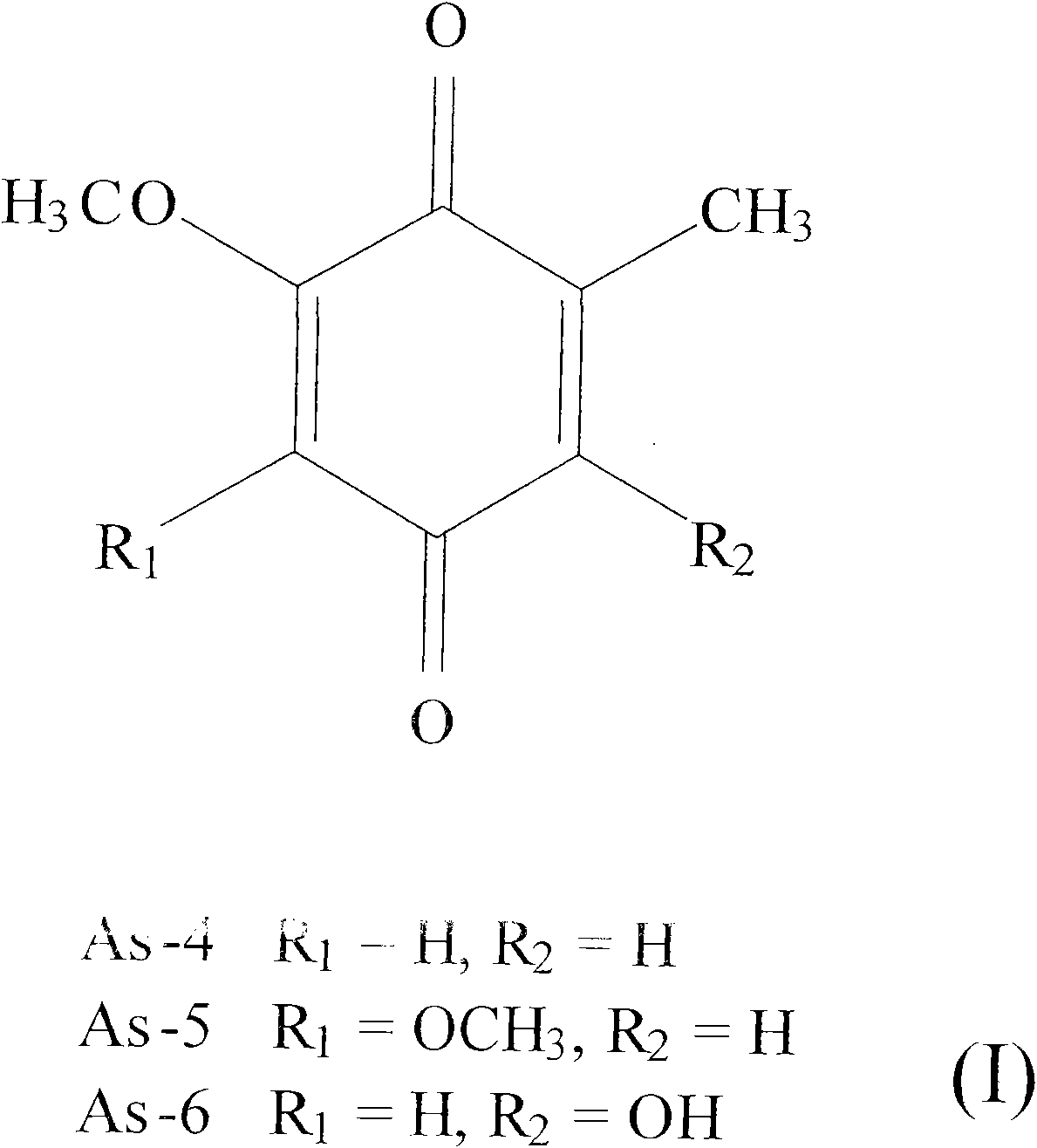
[0063] 1. Identification of the chemical structure of compound As-4
[0064] Dark yellow solid, MS (mass spectrometer, Finnigan GCQ) measured molecular weight of 152, by 1H-NMR and 13C-NMR (d-solvent: CDCl 3 ) (NMR instrument, Varian Unity Inova-500Spectrometer, 500MHz) measurement comparison, δH 2.07 (3H, d, J=1.5Hz) is the methyl signal at the C-7 (δC15.7) position, δH 3.82 ( 3H, s) is the methoxy signal at C-2 (δC 56.5), δH 5.88 (1H, d, J=2.5Hz) and δH 6.53 (1H, d, J=1.5Hz) are at C-3 (δC107.5) and C-5 (δC 134.1) position of the aromatic hydrogen signal, and then by HRMS (high resolution gas chromatography mass spec...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
| Concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com