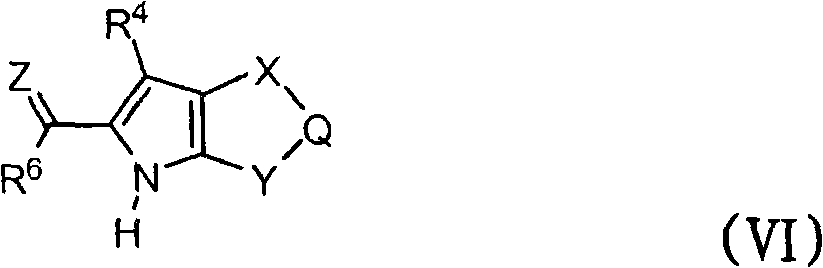Inhibitors of D-amino acid oxidase
An aryl, alkyl technology, applied in the field of D-amino acid oxidase inhibitors, can solve problems such as improving or enhancing learning, memory or cognition, etc. that are not mentioned
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
[0131] In another exemplary embodiment, the compound of the invention has a structure selected from:
where Z, A, R 4 , R 6 , R 1 and R 2 as defined in formula (I). In the above structures, each stereocenter marked with an asterisk "*" or "**" is independently racemic or as defined. In one example, a stereocenter marked with "*" has the (R)-configuration. In another example, a stereocenter marked with a "*" has the (S)-configuration. R 1 and R 2 Together with the atoms to which they are attached, optionally bonded to form a 3- to 7-membered ring.
[0132] In one embodiment, R 1 and R 2 combine to form substituted or unsubstituted cyclopropane rings. Exemplary compounds according to this embodiment have structures selected from the following formulae:
where R 30 and R 31 is a member independently selected from H, substituted or unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or unsubstituted aryl, substituted or unsubstituted he...
Embodiment 1
[0298] The title compound was synthesized from cyclopentanone according to general methods 1A, 1B and 1C. In the last step, 1.5 g (7.48 mmol) of ethyl (E)-3-(2-chlorocyclopent-1-enyl)acrylate were cyclized. The crude product was purified by flash chromatography (0-50% EtOAc / heptane) to provide 418 mg (31%, last step) of 1,4,5,6-tetrahydrocyclopenta[b]pyrrole- 2-Carboxylic acid ethyl ester. 1 H NMR (400MHz, CDCl 3 )δppm 1.34(t, J=7.13Hz, 3H), 2.38-2.48(m, 2H), 2.59-2.65(m, 2H), 2.69-2.75(m, 2H), 4.30(q, J=7.13Hz, 2H), 6.67 (d, J = 1.37 Hz, 1H), 8.78 (brs, 1H); LCMS-MS (ESI+) 179.9 (M+H).
[0299] According to General Procedure 7, ethyl 1,4,5,6-tetrahydrocyclopenta[b]pyrrole-2-carboxylate ( 100mg, 0.56mmol) to synthesize the title compound. The crude product was purified by flash chromatography (0-100% EtOAc / heptane) to give 61 mg (73%) of 1,4,5,6-tetrahydro-cyclopenta[b]pyrrole-2- Carboxylic acid (1). 1 H NMR (400MHz, CD 3 OD) δppm2.35-2.44 (m, 2H), 2.54-2.61 (m, 2H), 2...
Embodiment 2
Synthesis of Pyrrole Analogs Using 4-Substituted Fused Cyclopentanes
2.1.) Synthesis of methyl 4-oxo-1,4,5,6-tetrahydrocyclopentadieno[b]pyrrole-2-carboxylate
2.1.a) Synthesis of methyl 5-formyl-1H-pyrrole-2-carboxylate
[0300] To 1,2-dichloroethane (40 mL), DMF (13.6 mL, 176 mmol) was added. The mixture was cooled to 0 °C, and phosphorus oxychloride (16.4 mL, 176 mmol) was added dropwise over 5 minutes. The resulting solution was stirred for 15 minutes. To this solution was added methyl 1H-pyrrole-2-carboxylate (20 g, 160 mmol) in dichloroethane (80 mL) dropwise at 0 °C (ca. 1 h). The cooling bath was removed, and the reaction mixture was heated to reflux for about 1 hour, then allowed to cool to room temperature. EtOAc (250 mL) in ice water (400 mL) was added and the organic layer was separated. with NaHCO 3 The solution neutralized the aqueous layer, then eluted with EtOAc (4 x 100 mL). with dilute NaHCO 3 solution, brine washed organic layer and combined organic ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com