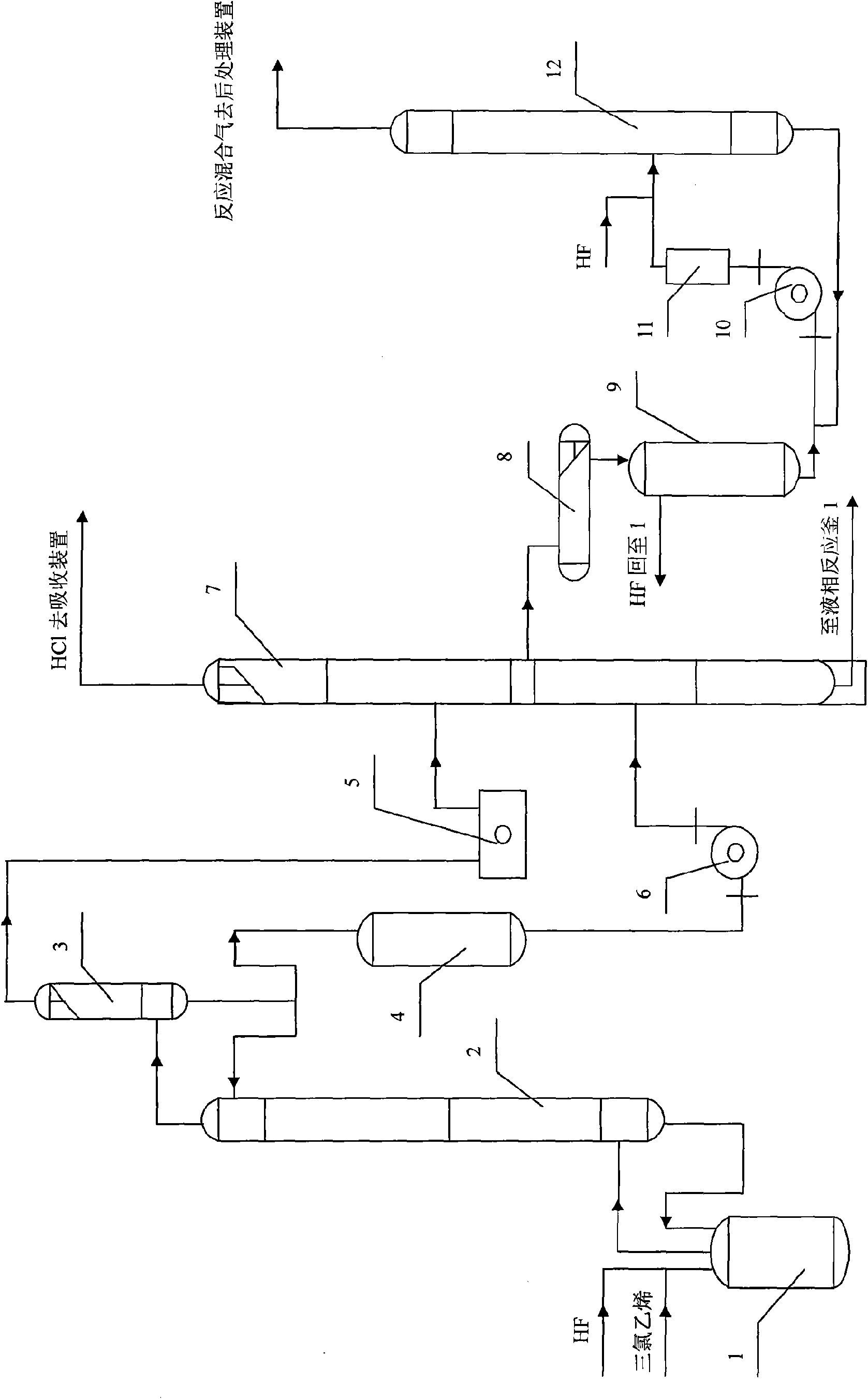
Producing process for synthesizing 1, 1, 1, 2-tetrafluoroethane by liquid phase-gas phase method
A kind of technology of tetrafluoroethane and production process, applied in the field of liquid phase-gas phase method synthesis 1
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
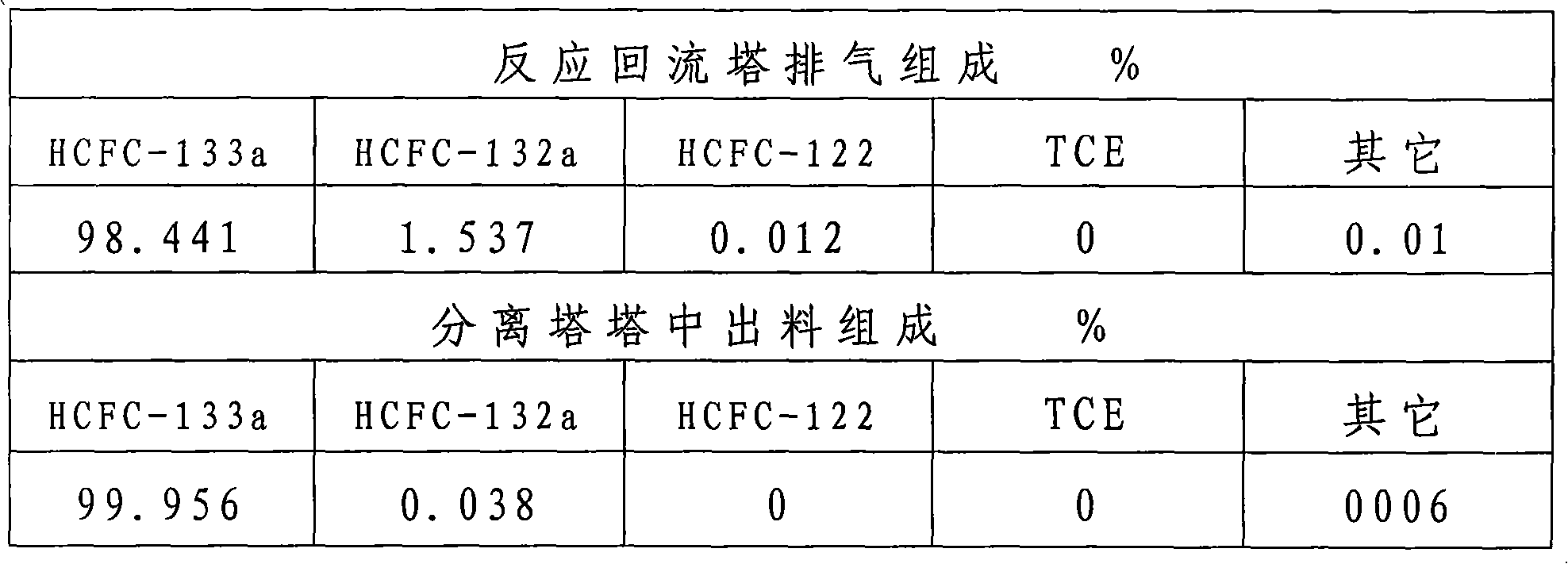
[0031] According to the above process flow and steps, 10KgSbCl is installed in a 50L liquid phase reactor 1 5And drop into 20KgHF after activating for 5 hours, then drop into TCE, HF continuously simultaneously, TCE, HF feeding mass ratio is 1.7: 1, and the feeding rate of TCE is 3Kg / h, and reaction temperature is controlled at 45 ℃, and reaction pressure is controlled at 0.5Mpa, The temperature at the top of the reaction reflux column 2 is controlled at 26°C. The top temperature of the separation tower 7 is controlled at -35°C, the tower pressure is 0.8MPa, and the discharge temperature in the tower is 50°C. After the operation is stable, the reflux exhaust, the discharge sampling chromatographic analysis results in the separation tower 7 are shown in Table 1.
[0032] Table I
[0033]
[0034] From the data in Table 1, it can be concluded that the conversion rate of TCE is close to 100%, and the HCFC-133a and HF azeotrope extracted from the separation tower have less im...
Embodiment 2
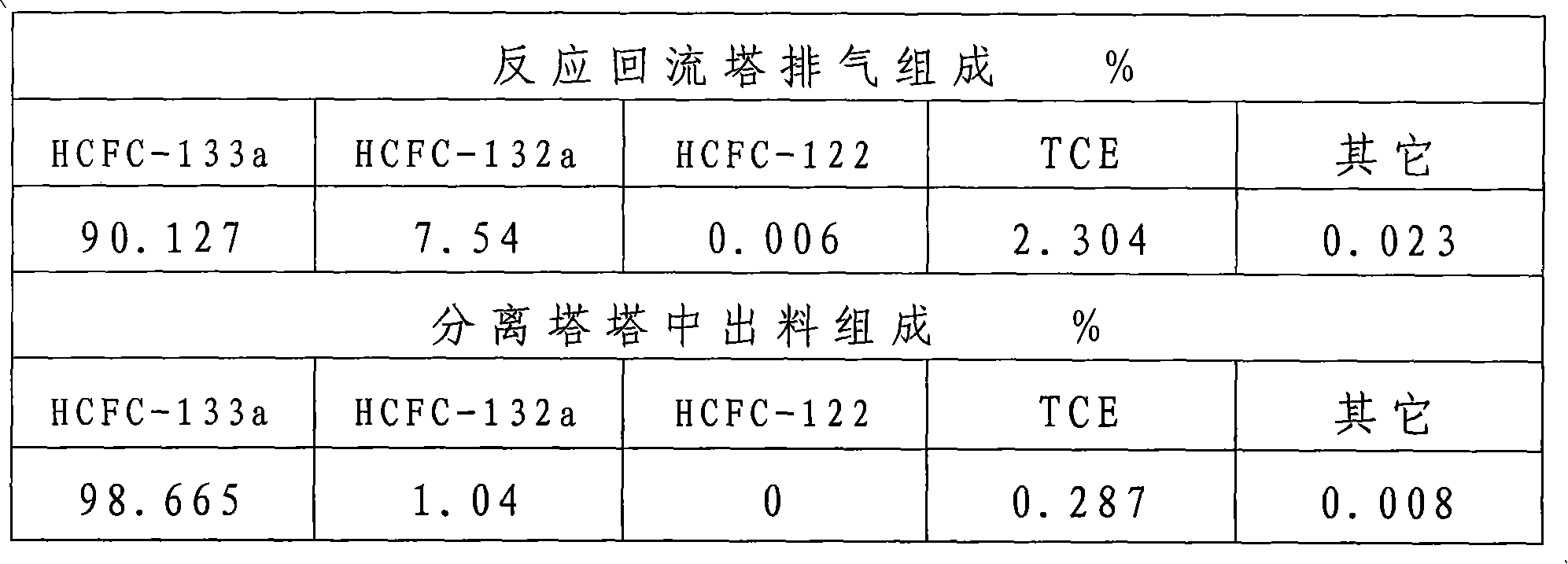
[0036] By the operating steps of Example 1, 1Kg SbCl is charged in the liquid phase reactor 1 5 And put in 20KgHF and other conditions remain unchanged, after the operation is stable, the reflux exhaust, the discharge sampling chromatographic analysis results in the separation tower are shown in Table 2.
[0037] Table II
[0038]
[0039] Conclusion: As can be seen from the data in Table 2, when other conditions remain unchanged, HF and catalyst SbCl 5 When the mass ratio of TCE is greater than 15, the conversion rate of TCE decreases.
Embodiment 3
[0041] By the operating steps of Example 1, 5Kg SbCl is charged in the liquid phase reactor 1 5 And put in 5KgHF and other conditions remain unchanged, after the operation is stable, the reflux exhaust, the discharge sampling chromatographic analysis results in the separation tower are shown in Table 3.
[0042] Table three
[0043]
[0044] Conclusion: According to the above data, when the mass of antimony pentachloride and hydrogen fluoride accounts for a small amount of materials in the reactor, the conversion rate of TCE decreases, which is not conducive to the liquid phase fluorination reaction.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com