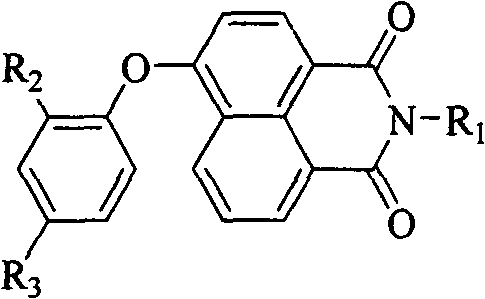
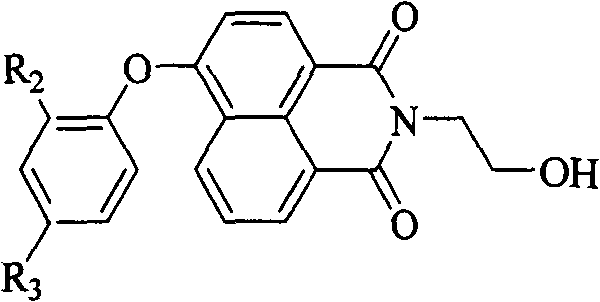
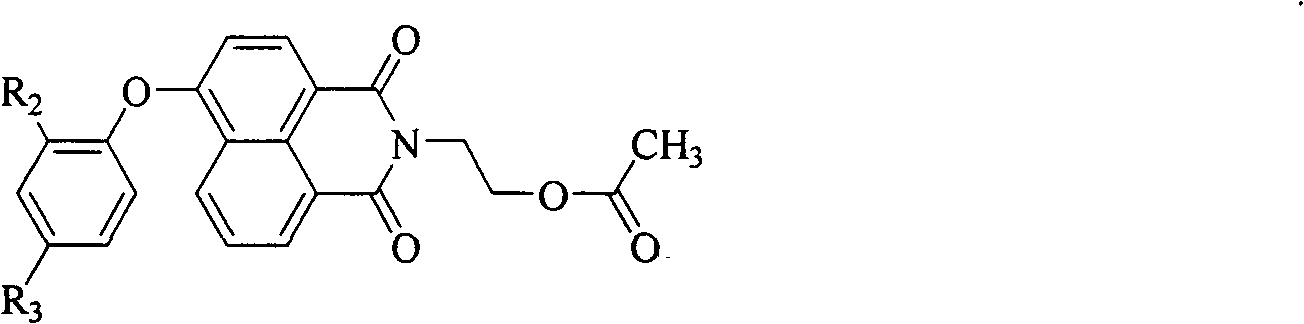
Blue light naphthalimide fluorescent compound
A naphthalimide and compound technology, which is applied in the field of naphthalimide compounds, can solve the problems of fluorescence quenching, device performance degradation, efficiency decline, etc., and achieve the effect of high solid-state fluorescence quantum efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] The naphthalimide derivative in this example is N-(2-hydroxyethyl)-4-(4-tert-butylphenoxy)-1,8-naphthalimide (PONA1). The fluorescence of its solution is blue, and the fluorescence emission is at 429nm; the solid fluorescence is blue-green, and the fluorescence emission is at 471nm. By introducing a large sterically hindered tert-butyl group to the 4-position of 4-phenoxy, the purpose of increasing the molecular volume, reducing the intermolecular force, and improving the quantum yield of solid fluorescence is realized. The quantum efficiency of solid fluorescence is 41.56%. . The preparation process of the target compound described in this example includes: 1) Aminolysis reaction with 2-ethanolamine with 4-bromo-1,8-naphthalene dicarboxylic anhydride as the starting material; 2) Substitution reaction with phenol derivatives There are two steps.
[0043] 1) Amination reaction of 4-bromo-1,8-naphthalene dicarboxylic anhydride and 2-ethanolamine to generate N-(2-hydroxy...
Embodiment 2
[0050] The naphthalimide derivative in this example is N-(2-hydroxyethyl)-4-(2-tert-butylphenoxy)-1,8-naphthalimide (PONA2). The fluorescence of its solution is blue, and the fluorescence emission is at 432nm; the solid fluorescence is pure blue, and the fluorescence emission is at 448nm. By introducing a large sterically hindered tert-butyl group to the 2-position of 4-phenoxy group, the coplanarity of the benzene ring, oxygen atom and naphthalimide ring can be reduced, and the degree of conjugation can be reduced, so that the solid-state fluorescence emission spectrum The purpose of tuning from teal to pure blue. The preparation process of the target compound described in this example is the same as that in Example 1. Its reaction formula is as follows:
[0051]
[0052] The synthesis method is the same as in Example 1, except that the raw material is changed from 4-tert-butylphenol to 2-tert-butylphenol. The crude product was purified by column chromatography (eluent:...
Embodiment 3
[0054] The naphthalimide derivative in this example is N-(2-hydroxyethyl)-4-(2,4-di-tert-butylphenoxy)-1,8-naphthalimide (PONA3). The fluorescence of its solution is blue, and the fluorescence emission is at 432nm; the solid fluorescence is pure blue, and the fluorescence emission is at 448nm. By introducing two large sterically hindered tert-butyl groups to the 2- and 4-positions of 4-phenoxy, the following simultaneously can be achieved: 1) increasing molecular volume, reducing intermolecular forces, and improving solid fluorescence quantum yield; 2) The purpose of reducing the coplanarity of the benzene ring, the oxygen atom and the naphthalimide ring, and reducing the degree of conjugation, so that the solid fluorescence emission spectrum is tuned from blue-green to pure blue. Its solid fluorescence quantum efficiency is 28.59%. The preparation process of the target compound described in this example is the same as that in Example 1. Its reaction formula is as follows: ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com