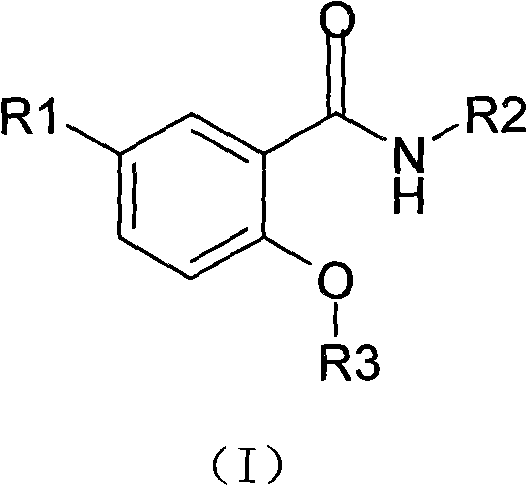
3-substituted salicylamide compound, preparation method, medicinal composition and application thereof
A technology of salicylamide and compounds, applied in the field of 3-substituted salicylamide derivatives
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
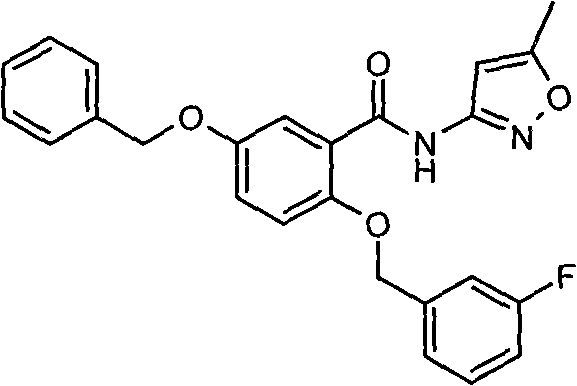
[0276] Example 1.2-(3-fluorobenzyloxy)-5-benzyloxy-N-(5-methylisoxazol-3-yl)-benzamide
[0277]
[0278] Synthesis of 5-benzyloxy-2-hydroxybenzoic acid:
[0279] A DMF solution of 10ml 2,5-dihydroxygentisic acid (3.082 grams, 0.02mol) was added dropwise to 10ml of DMF suspension containing 60% NaH (1.761 grams, 0.044mol), a large amount of gas was produced, and at room temperature After stirring for 2 hours, a solution of benzyl bromide (3.421 g, 0.02 mol) in 7 ml of DMF was added dropwise to the suspension. After continuing to stir for 2-3 hours, the reaction solution was poured into 60 ml of distilled water, and the pH value was adjusted to 2 with concentrated HCL. A large amount of solids were precipitated. The solids were filtered out and recrystallized with chloroform to obtain 2.347 g of white crystals, which were recrystallized. After the mother liquor was spin-dried, 1.317 g of white crystals were obtained by solid column chromatography. 1 H-NMR (Acetone-d 6 , 30...
Embodiment 2
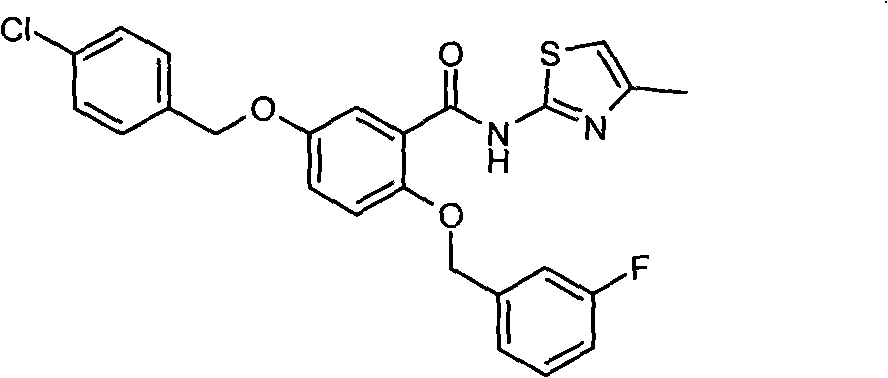
[0284] Example 2.2-(3-fluorobenzyloxy)-5-(4-chlorobenzyloxy)-N-(4-methylthiazol-2-yl)-benzamide
[0285]
[0286] Synthesis of 5-(4-chlorobenzyloxy)-2-hydroxybenzoic acid:
[0287] A DMF solution of 10ml 2,5-dihydroxygentisic acid (3.082 grams, 0.02mol) was added dropwise to 10ml of a DMF suspension containing 60% NaH (1.761 grams, 0.044mol), and after stirring for 2 hours, 7ml1 -Chloro-4-(chloromethyl)benzene (3.221 g, 0.02 mol) in DMF was added dropwise to the brown suspension. After 4 hours, the reaction solution was poured into 60 ml of distilled water, and the pH value was adjusted to 3 with concentrated HCL. A large amount of solids were precipitated, and the filtered solids were separated by column chromatography to obtain 3.34 g of white crystals. 1 H-NMR (Acetone-d 6 , 300M) δ5.11 (s, 2H, ph-CH 2 -O-), 6.91(d, 1H, ArH), 7.25(dd, 1H, ArH), 7.40-7.53(m, 5H, ArH), 10.68(s, 1H, OH), 12.05(s, 1H, - OH). MS FAB : (M+1) + =279
[0288] Synthesis of 2-(3-fluorobenz...
Embodiment 3
[0292] Example 3.5-(4-chlorobenzyloxy)-2-isopropoxy-N-(pyridin-2-yl)-benzamide
[0293]
[0294] Synthesis of 5-(4-chlorobenzyloxy)-2-isopropoxybenzoic acid:
[0295] Dissolve 5-(4-chlorobenzyloxy)-2-hydroxybenzoic acid (1.393 g, 5 mmol) in 30 ml DMF, add anhydrous K 2 CO 3 (1.656 g, 12 mmol), stirred at 60 °C for 1 hour, returned to room temperature, added 2-bromopropane (1.353 g, 11 mmol) dropwise to the reaction solution, stirred at 150 °C for 6 hours, then added anhydrous K 2 CO 3 (0.414 grams, 3mmol), 2-bromopropane (0.615 grams, 5mmol), stirred at 150°C for another 6 hours, stopped heating, and suction filtered after cooling down to room temperature, and the filtrate was decompressed to spin off most of the solvent to obtain a viscous liquid , add ethyl acetate and distilled water to dissolve, extract and wash with water, dry the organic layer, and spin dry the ethyl acetate to obtain about 1.5 grams of viscous, which is the crude product 5-(4-chlorobenzyloxy)-2-is...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com