New process for synthesizing tenofovir disoproxil fumarate
A technology of tenofovir dipivoxil and a new process, which is applied in the field of preparation of antiviral drug tenofovir dipivoxil, can solve the problems of not reaching clinical drug use, unstable yield of chiral enrichment treatment, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
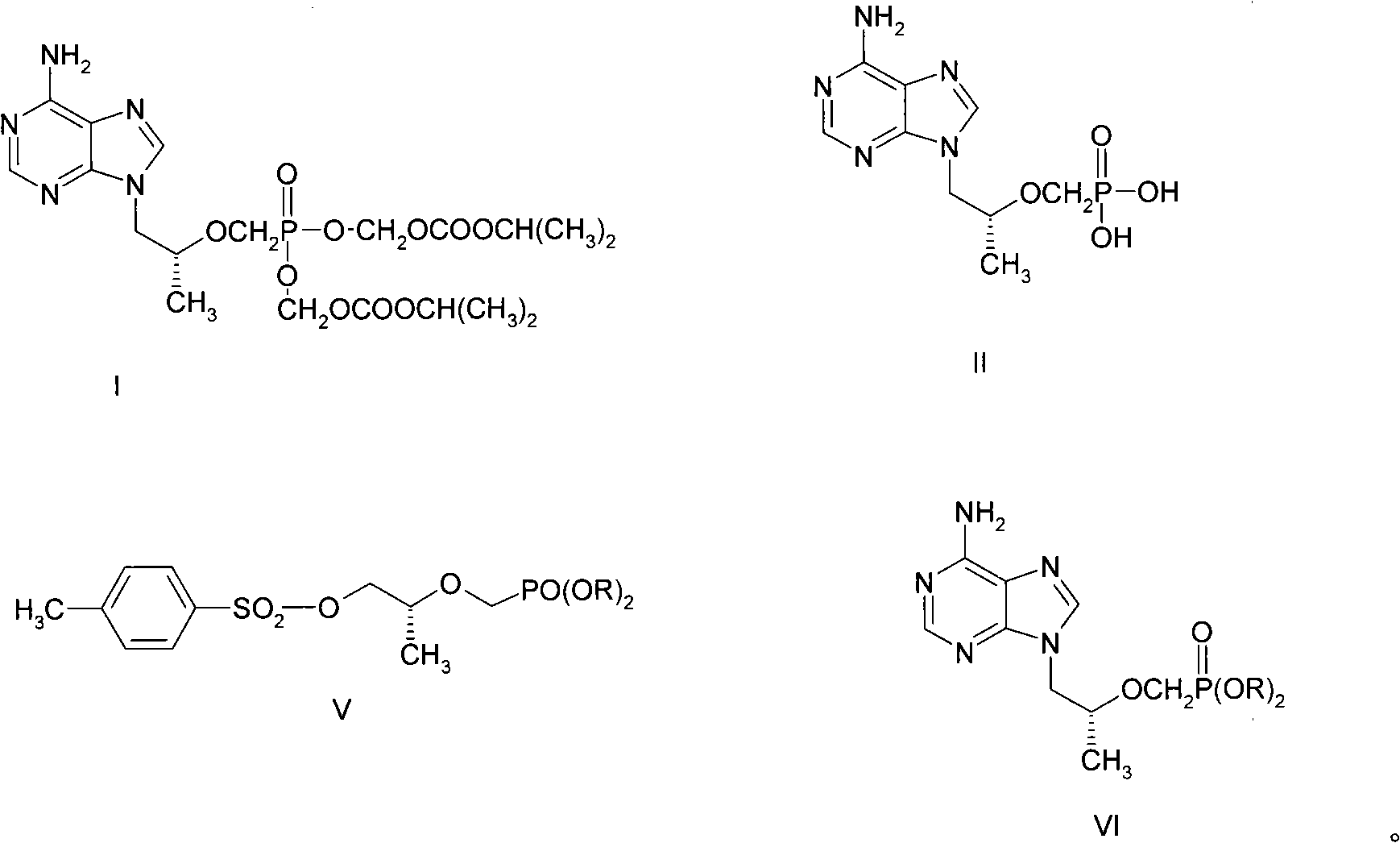
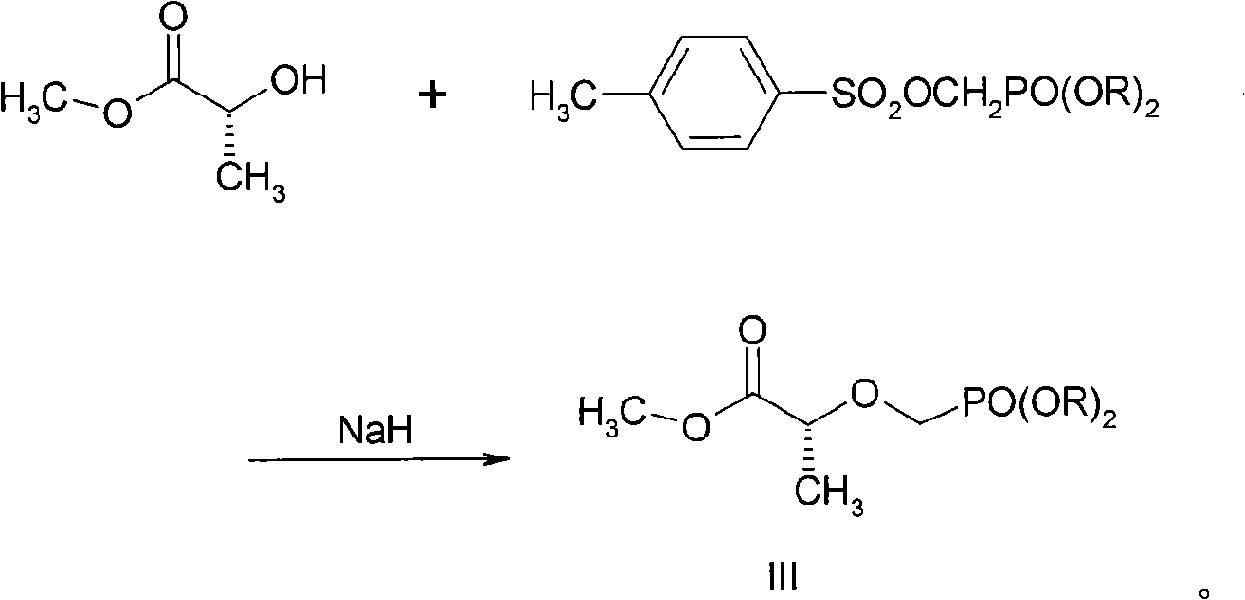
[0025] Example 1 Preparation of 2-O-(diisopropyloxy-phosphono-methyl)-(R)-lactate methyl ester (III)
[0026] 1.1 Synthesis of diisopropyl p-toluenesulfonyloxyphosphonate
[0027] The synthetic route is as follows:
[0028] PCl 3 +(CH 3 ) 2 CHOH→HPO[OCH(CH 3 ) 2 ] 2
[0029] (HCHO) n +HPO[OCH(CH 3 ) 2 ] 2 →HOCH 2 PO[OCH(CH 3 ) 2 ] 2
[0030]
[0031] Under ice bath, 787g (5.73mol) PCl 3 Slowly added dropwise to 1033g (17.2mol) of isopropanol, the dropwise addition was completed in 2 hours, and continued stirring under ice bath for 30 minutes. The ice bath was removed and stirred at room temperature for 3 hours. The low-boiling point solvent was evaporated by rotary evaporation, fractionated under reduced pressure, and the components at 70°C / 2mmHg were collected to obtain 450 g of diisopropyl phosphonite.
[0032] Add 12ml of toluene, 80g (0.48mol) of diisopropyl phosphite, 22g of paraformaldehyde and 6g of triethylamine in a nitrogen-filled reaction vesse...
Embodiment 2
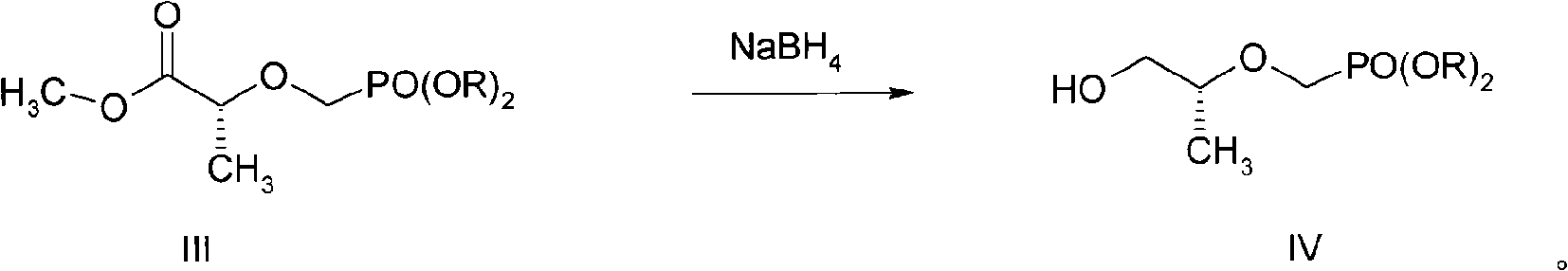
[0035] Example 2 Preparation of 2-O-(diisopropyloxy-phosphono-methyl)-(R)-propanol (IV)
[0036] Add 14.2g (50.3mmol) of compound III to 840ml of anhydrous THF, under stirring in an ice bath, add 4.53g (120mmol) of NaBH in batches 4 , reacted in an ice bath for 15 minutes, removed the ice bath, and continued to react at room temperature for 8 hours (determining the reaction situation by TLC detection), until the end of the reaction.
[0037] Filtration, the filtrate was rotary evaporated to remove the solvent, extracted with ethyl acetate, anhydrous Na 2 SO 4 Dry overnight, filter, remove the solvent by rotary evaporation, use ethyl acetate as the eluent, and separate by column chromatography to obtain 6.82 g of light yellow oily liquid of IV, with a yield of 53.3%. H-NMR (DMSO, δppm): 4.583-4.640 (m, 2H); 3.761-3.783 (d, 2H); 3.492-3.507 (m, 1H); 3.383-3.396, 3.298-3.345 (m, 2H); 1.237-1.253 (m, 12H); 1.047-1.062 (dd, 3H).
Embodiment 3
[0038] Example 3 Preparation of 2-O-(diisopropyloxy-phosphono-methyl)-(R)-propyl p-toluenesulfonate (V)
[0039]In a 500ml three-neck flask, 110ml of pyridine, 13g (51mmol) of compound IV and 0.7ml of dimethylaminopyridine were added successively, and cooled in an ice bath. A solution of 36 g (188.8 mmol) of TsCl dissolved in 60 ml of pyridine was slowly added dropwise under stirring, and reacted overnight in an ice bath. Distilled water was added, the solvent was removed by rotary evaporation, extracted with ethyl acetate, washed with water, then acidified with 1M HCl, again with water, saturated NaHCO 3 , wash. The organic layer was separated and washed with anhydrous MgSO 4 dry. After filtration, the filtrate was rotary evaporated to remove the solvent to obtain 13.8 g of V as a yellow oily liquid, 66.25%. H-NMR (CDCl 3 , δppm): 7.783-7.804 (d, 2H); 7.274-7.367 (d, 2H), 4.712-4.731 (m, 2H); 3.960 (d, 2H); 3.776-3.822 (m, 2H); 3.697-3.753 (m, 2H); 2.456 (s, 3H); 1.296-...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| optical purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com