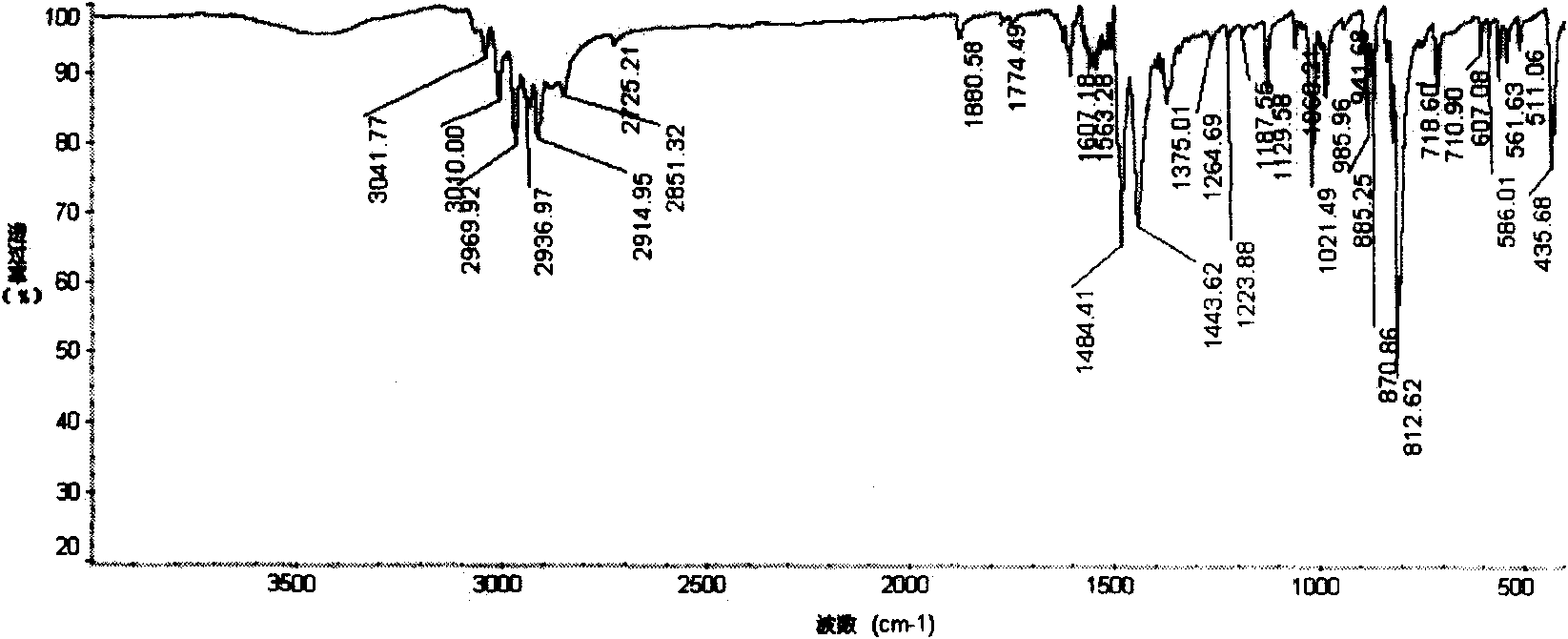
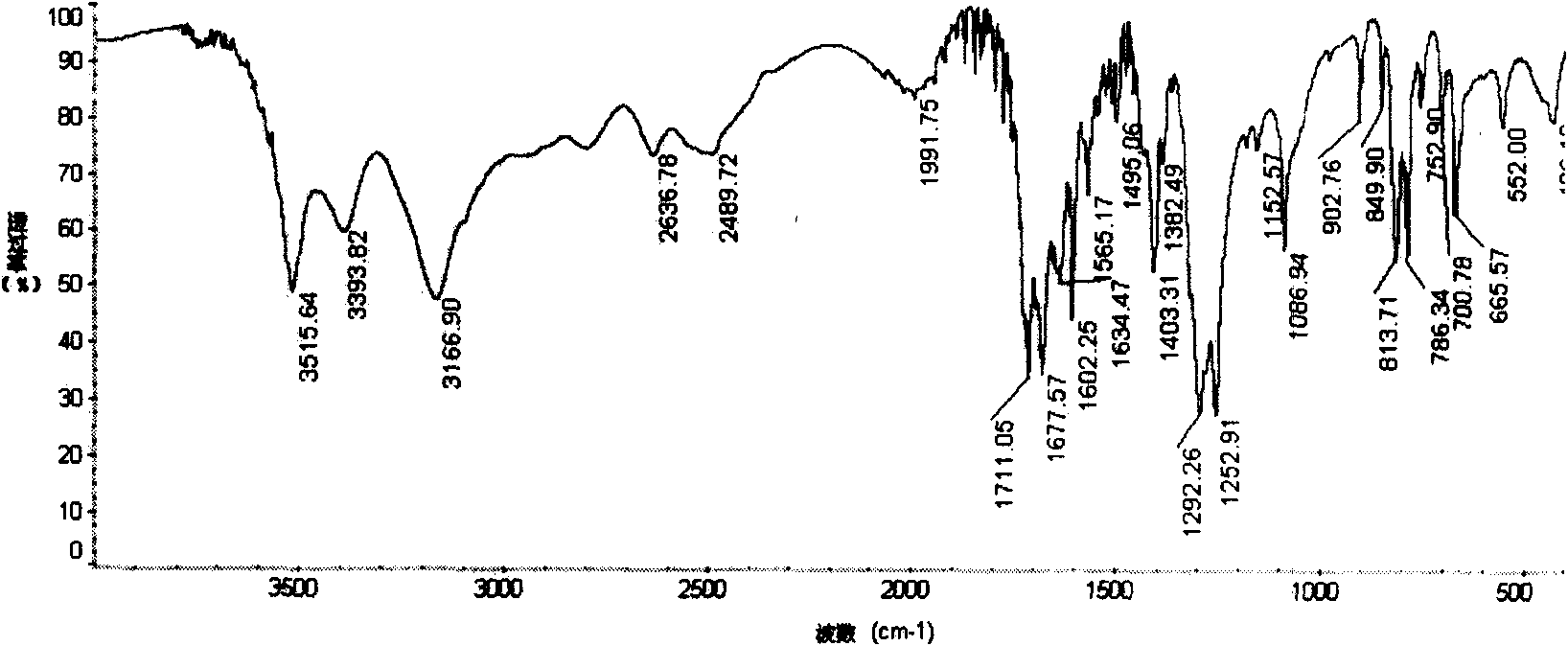
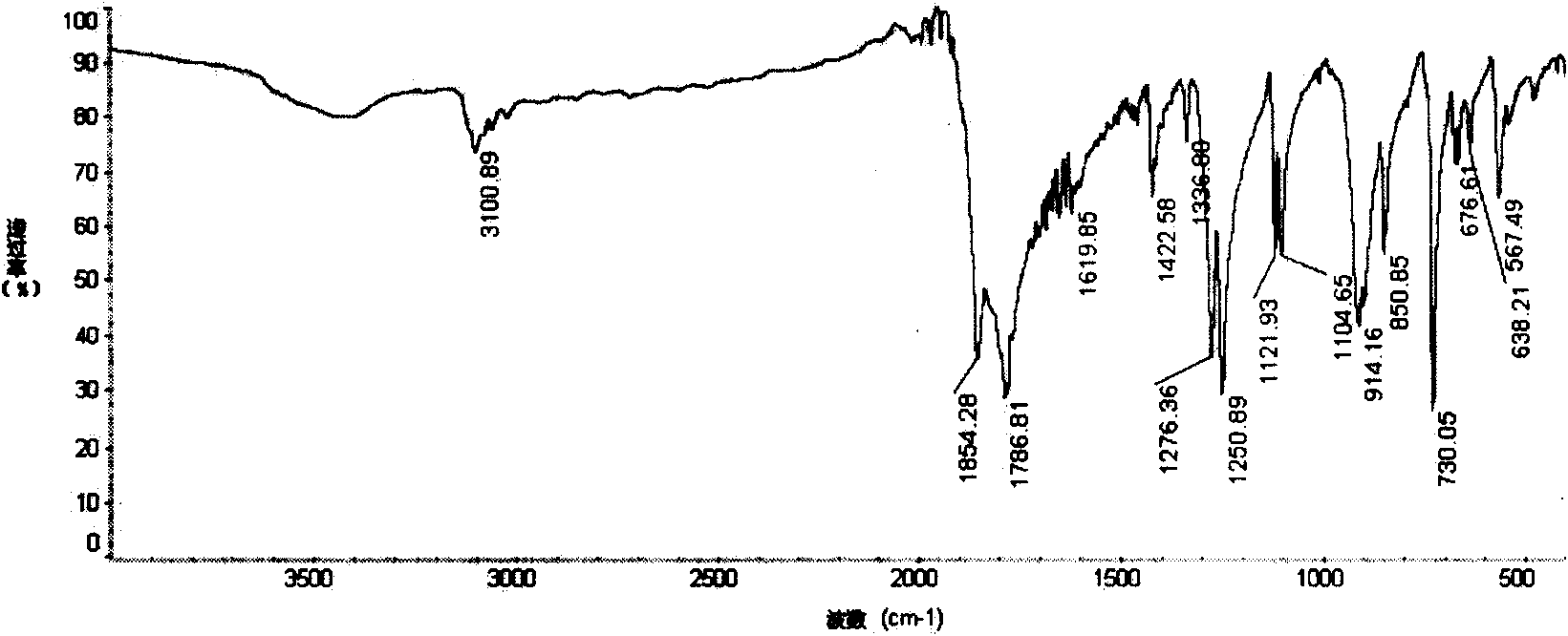
Method for preparing diphenyl tetracarboxylic dianhydride
A technology of biphenyltetracarboxylic dianhydride and biphenyltetracarboxylic acid, which is applied in the field of preparing biphenyltetracarboxylic dianhydride, can solve the problems of harsh equipment and reaction conditions, difficulty in large-scale production, etc., and achieve low price and simple production process Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0015] A kind of preparation method of biphenyltetracarboxylic dianhydride comprises following reaction:
[0016] Halogenation reaction: using o-xylene as a raw material for halogenation to obtain halogenated xylene;
[0017] Coupling reaction: 3,3',4,4'-tetramethylbiphenyl is prepared by coupling halogenated xylene;
[0018] Oxidation reaction: 3,3',4,4'-tetramethylbiphenyl is oxidized to produce 3,3',4,4'-biphenyltetracarboxylic acid;
[0019] Dehydration reaction: 3,3',4,4'-biphenyltetracarboxylic acid is dehydrated to obtain 3,3',4,4'-biphenyltetracarboxylic dianhydride.
[0020] The halogenation reaction is a reaction in the presence of a halogen and a catalyst using o-xylene as a raw material.
[0021] The catalyst is iron and iodine, wherein the molar ratio of iron and iodine is greater than zero and less than or equal to 1:1, wherein 0.1:1, 0.5:1, and 1:1 all have good effects.
[0022] In the halogenation reaction, the molar ratio of o-xylene, catalyst and halogen ...
Embodiment 1
[0040] (1) Preparation of halogenated o-xylene by halogenation reaction
[0041]
[0042] Add 500 g (569 ml, 4.72 moles) of o-xylene, 2.5 grams of iron powder and 2.5 grams of iodine into a 1000ML four-necked bottle. Install a dropping funnel, a stirrer, a condenser tube, a thermometer, and connect a gas absorption device to the top of the condenser tube. The reaction mixture was stirred and cooled to -5°C-5°C under an ice bath, and 660 g (4.13 moles) of liquid bromine was slowly added into the reaction flask, and the addition was controlled within 3 hours. During this period, the temperature of the reaction solution was always controlled at -5°C-5°C. After all the liquid bromine was added, the temperature of the reaction solution was allowed to rise to room temperature, and stood still overnight. Then the reaction solution was poured into 500ML water, stirred and separated. Then wash twice with 3% sodium hydroxide solution. Then wash twice with 500ML water, separate the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com