Application of astragaloside in treating delayed type hypersensitivity mediated diseases
A technology of astragaloside IV and hypersensitivity, which is applied in the field of medicine, can solve the problems of prevention and treatment of diseases mediated by astragaloside IV, and achieve the reduction of pathological and histological damage and inflammation The effect of the reaction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
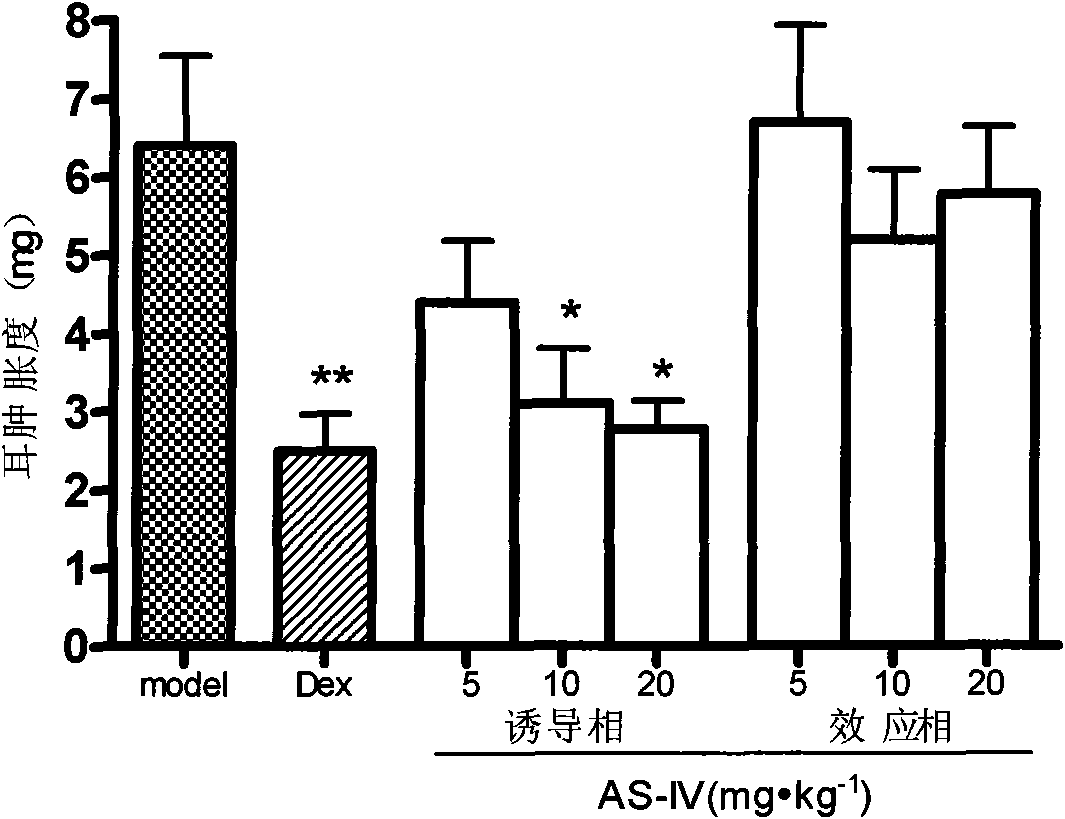
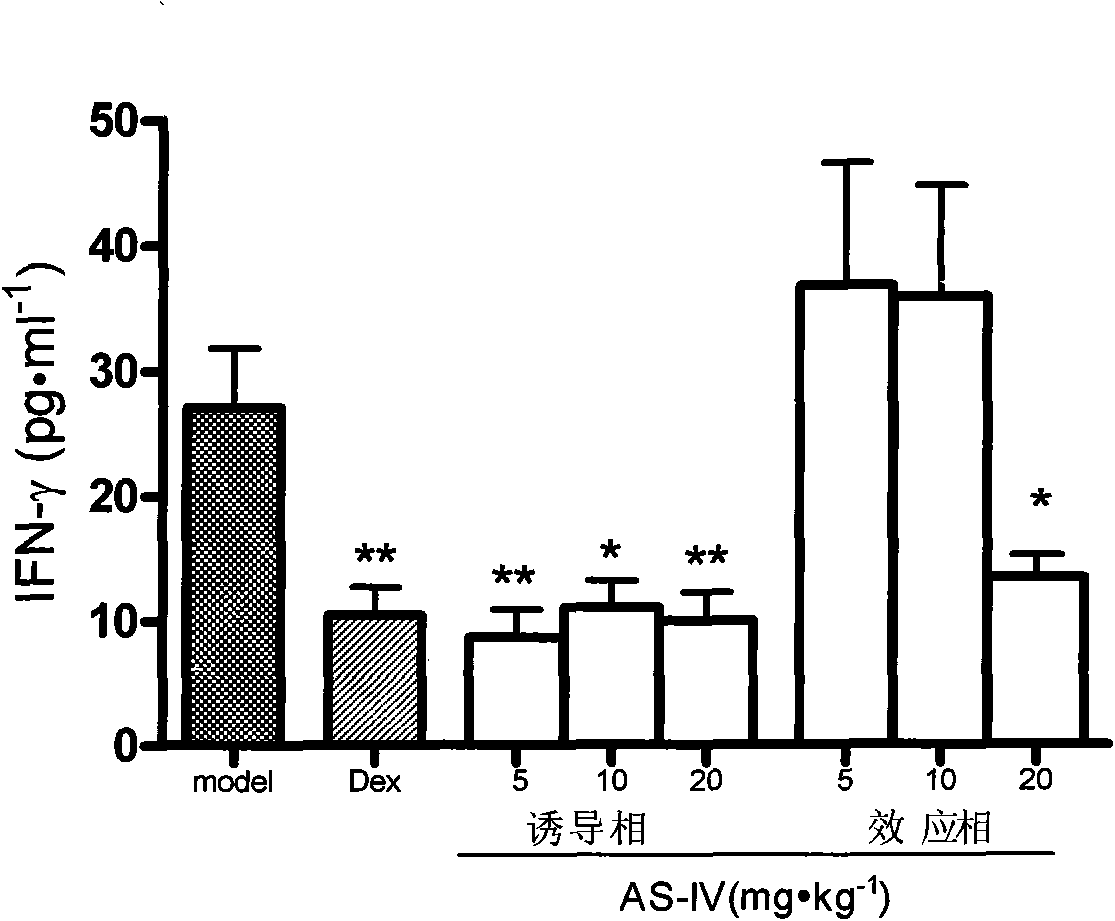
[0033] Example 1 The inhibitory effect of astragaloside IV on delayed-type hypersensitivity has the following experimental evidence:
[0034] Effect of Astragaloside IV on Allergic Contact Dermatitis Mouse Model
[0035] Methods: ICR clean level mice were adaptively fed for 3 days and divided into 8 groups, namely model group, dexamethasone group (0.65mg / kg), astragaloside IV induction phase administration (5mg / kg group, 10mg / kg kg group, 20mg / kg group), astragaloside IV effect administration (5mg / kg group, 10mg / kg group, 20mg / kg group). The administration of astragaloside IV (intraperitoneal injection) in the induction phase was administered daily from the first day of modeling, and the administration was stopped on the day before the challenge; the administration of astragaloside IV (intraperitoneal injection) in the effect phase was administered at 0h and 5h after the challenge , 9h, 18h and 24h administration; the dexamethasone group was administered intraperitoneally eve...
Embodiment 2
[0042] Example 2 Application of Astragaloside IV in the Preparation of Drugs for the Treatment of Delayed Hypersensitivity Diseases
[0043] The dosage of astragaloside IV in the present invention varies due to different dosage forms. The general dosage is 50mg-400mg orally per person per day, and the dosage for injection is 50mg-150mg. (estimated from animal dose)
[0044] When the medicine of the present invention is used as an anti-delayed hypersensitivity disease, it can be administered through the skin, mucous membranes, enteral and parenteral, and can be made into solid form tablets, capsules, granules, powders, or Ointment or ointment in semi-solid form, or powdered injection, or dosage forms such as injection, suspension, emulsion or liniment in liquid form for clinical application. The above-mentioned medicines in various dosage forms can be prepared according to conventional methods in the field of pharmacy. Delayed hypersensitivity diseases include contact dermati...
Embodiment 3
[0046] Example 3 Preparation of Astragaloside IV Sodium Chloride Injection
[0047] Weigh 10g of astragaloside IV, add 2L each of ethanol and propylene glycol in turn, stir to dissolve, add 900g of sodium chloride, add water for injection to 100L, mix well, filter and sterilize, sub-package, 100ml per bottle, potting, to obtain 10mg / 100ml sterilized Astragaloside IV Sodium Chloride Injection.
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com