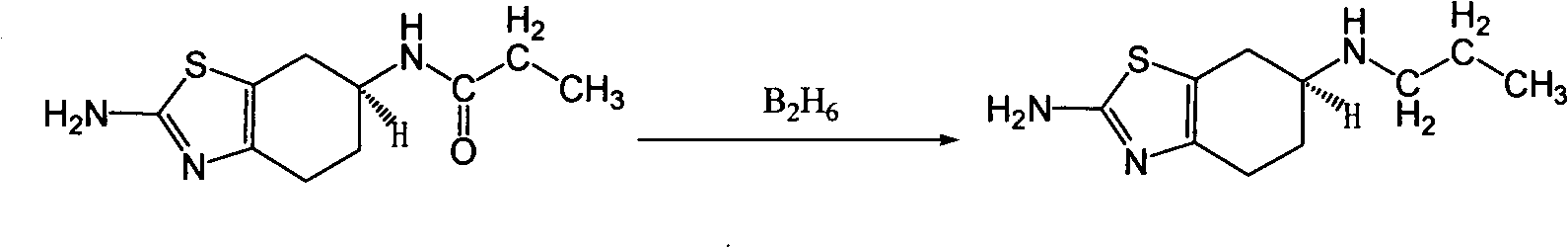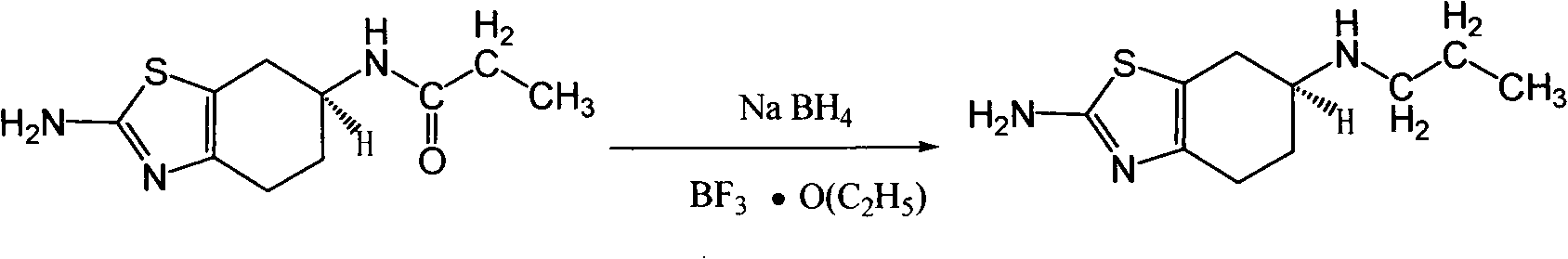Preparation method of pramipexole
An amino, tetrahydrogen technology, applied in the field of pramipexole, can solve the problems of unfavorable industrial production, irritating odor, harsh reaction conditions, etc., and achieve the effects of easy control, improved safety, and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] 10 L of tetrahydrofuran and 1.2 kg of sodium borohydride were successively added into a 20 L three-necked flask, and stirred for 1 hour. At T=25°C, 1 kg of (±) 2-amino-6-propionylamino-4,5,6,7-tetrahydrobenzothiazole was added, stirred for 4 hours, and heated to reflux for 48 hours. Natural cooling to 20 ℃ ~ 25 ℃. Slowly pour the reaction solution into 8 L of ice water, and add 6.4 kg of 37% hydrochloric acid dropwise at T=10°C to 20°C. After dropping, PH=1. After suction filtration, a colorless transparent liquid was obtained, and tetrahydrofuran was spin-off under reduced pressure to constant weight. After spinning, adjust the pH to 14 with 50% aqueous sodium hydroxide solution, and consume 7 L of aqueous sodium hydroxide solution. Stir for 1 hour. After suction filtration, the solid was air-dried at 50° C. to constant weight to obtain 870 g of off-white solid (±) 2-amino-6-propylamino-4,5,6,7-tetrahydrobenzothiazole, with a yield of 93%.
[0018] ( 1 H-NMR (400...
Embodiment 2
[0020] 10 L of tetrahydrofuran and 1.2 kg of sodium borohydride were successively added into a 20 L three-necked flask, and stirred for 1 hour. Add (-) 2-amino-6-propionylamino-4,5,6,7-tetrahydrobenzothiazole 1kg at T=25°C, stir for 4 hours, and heat to reflux for 48 hours. Natural cooling to 20 ℃ ~ 25 ℃. Slowly pour the reaction solution into 8 L of ice water, and add 6.4 kg of 37% hydrochloric acid dropwise at T=10°C to 20°C. After dropping, PH=1. After suction filtration, a colorless transparent liquid was obtained, and tetrahydrofuran was spin-off under reduced pressure to constant weight. After spinning, adjust the pH to 14 with 50% aqueous sodium hydroxide solution, and consume 7 L of aqueous sodium hydroxide solution. Stir for 1 hour. Suction filtration, and the solid was air-dried at 50° C. to constant weight to obtain 870 g of off-white solid (-) 2-amino-6-propylamino-4,5,6,7-tetrahydrobenzothiazole with a yield of 93%.
[0021] ( 1 H-NMR (400MHz, DMSO); δ(ppm):...
Embodiment 3
[0023] 10 L of tetrahydrofuran and 1.2 kg of lithium aluminum hydride were successively added into a 20 L three-necked flask, and stirred for 2 hours. When T=28°C, 1 kg of (±) 2-amino-6-propionylamino-4,5,6,7-tetrahydrobenzothiazole was added, stirred for 5 hours, and heated to reflux for 48 hours. Natural cooling to 20 ℃ ~ 25 ℃. Slowly pour the reaction solution into 8 L of ice water, and add 6.4 kg of 37% hydrochloric acid dropwise at T=10°C to 20°C. After dropping, PH=1. After suction filtration, a colorless transparent liquid was obtained, and tetrahydrofuran was spin-off under reduced pressure to constant weight. After spinning, adjust the pH to 14 with 50% aqueous sodium hydroxide solution, and consume 7 L of aqueous sodium hydroxide solution. Stir for 1 hour. After suction filtration, the solid was air-dried at 50° C. to constant weight to obtain 870 g of off-white solid (±) 2-amino-6-propylamino-4,5,6,7-tetrahydrobenzothiazole, with a yield of 93%.
[0024] ( 1 H...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com