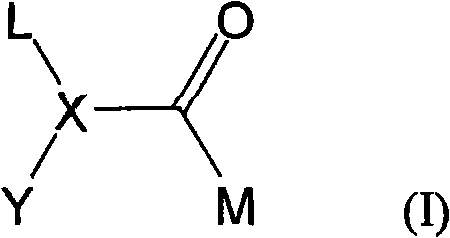
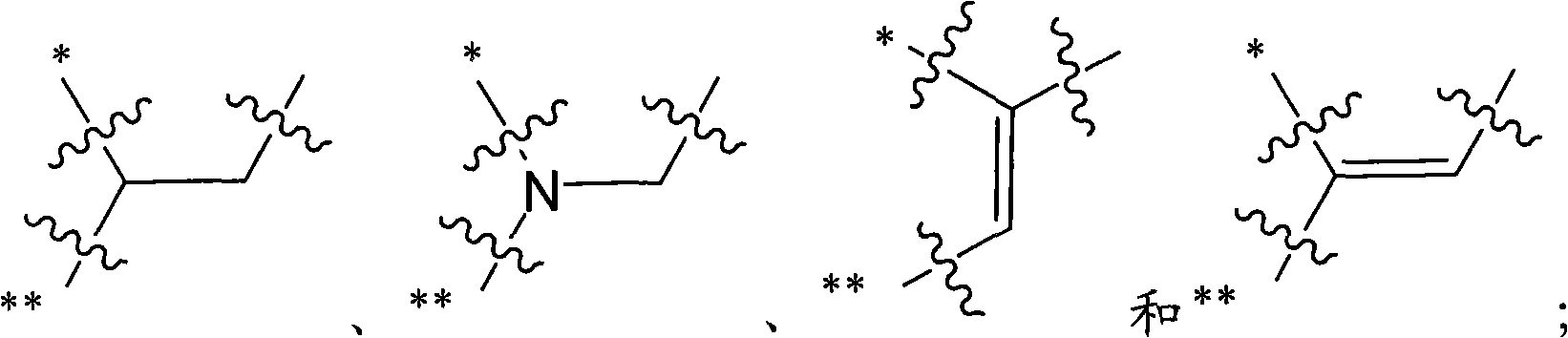
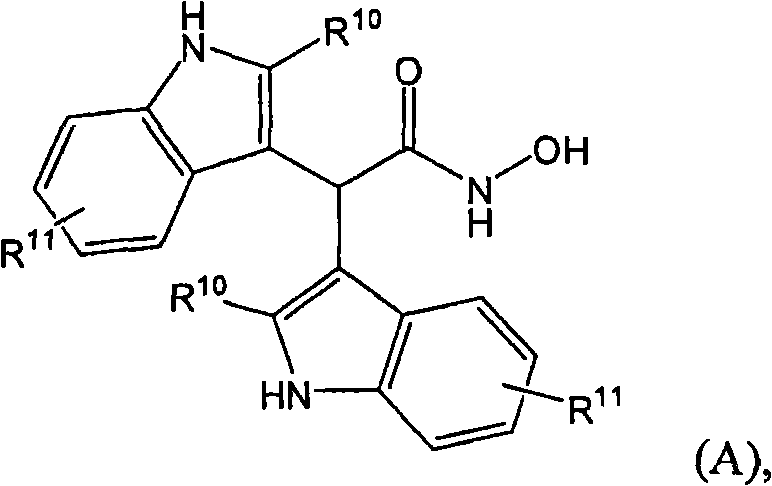
Inhibitors of histone deacetylase
A solvate, alkyl technology, applied in the field of compounds that inhibit histone deacetylase
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0291] 2,2-Bis(2,3-Dihydrobenzofuran-5-yl)-N-hydroxyacetamide (3)
[0292] plan 1
[0293]
[0294] Step 1: Methyl 2,2-bis(2,3-dihydrobenzofuran-5-yl)acetate (2)
[0295] To a solution of 1 (992 mg, 3.35 mmol) in methanol (20 mL), concentrated sulfuric acid (2 mL) was added, and the mixture was stirred at 60°C for 15 hours. The solvent was removed under vacuum. The crude material was dissolved in ethyl acetate and washed with water. Separate the organic layer and use Na 2 SO 4 It was dehydrated, dried, filtered, and concentrated to obtain compound 2 as a white solid (2.25 g, 94%). 1 H NMR(DMSO-d 6 )δ(ppm): 7.13(d, J=1.3Hz, 2H), 6.99(dd, J=8.2, 1.8Hz, 2H), 6.68(d, J=8.2Hz, 2H), 5.00(s, 1H) , 4.48 (t, J=8.8 Hz, 4H), 3.63 (s, 3H), 3.12 (t, J=8.8 Hz, 4H).
[0296] Step 2: 2,2-bis(2,3-dihydrobenzofuran-5-yl)-N-hydroxyacetamide (3)
[0297] To a solution of 2 (380 mg, 1.22 mmol) and hydroxylamine (50% in water, 1.25 ml) in 1:1 THF: methanol (10 ml), add alkali (KOH (275 mg, 4.9 mmol) or ...
Embodiment 2
[0299] N-hydroxy-2-phenoxy-2-phenylacetamide (6)
[0300] Scenario 2
[0301]
[0302] Step 1: Ethyl 2-phenoxy-2-phenylacetate (5)
[0303] To a solution of 4 (0.5 g, 2.06 mmol) and phenol (194 mg, 2.06 mmol) in THF (15 mL), add alkali (NaH (60% dispersion, 90 mg, 2.27 mmol) or three Ethylamine) and stirred at room temperature for 2 hours. The reaction mixture was quenched with water, diluted with ethyl acetate, and washed with water. Separate the organic layer and use Na 2 SO 4 Dehydrate, dry, filter, and concentrate. The crude material was purified by silica gel column chromatography with a gradient of ethyl acetate (0-25%) in hexane to obtain 5 as a colorless oil (140 mg, 27%). 1 H NMR(DMSO-d 6 )δ(ppm): 7.55-7.52(m, 2H), 7.44-7.34(m, 3H), 7.30-7.25(m, 2H), 6.96-6.92(m, 3H), 5.96(s, 1H), 4.13 -4.05 (m, 2H), 1.09 (t, J=7.0 Hz, 3H).
[0304] Alternative reaction conditions:
[0305] Compound 4 (1 equivalent), ROH (1 equivalent), K 2 CO 3 (1.5 equivalents), acetone, 50°C, 18 hours. ...
Embodiment 3
[0311] N-hydroxy-2,2-bis(4-nitrophenyl)acetamide (9)
[0312] Scheme 3
[0313]
[0314] Step 1: Methyl 2,2-bis(4-nitrophenyl)acetate (8)
[0315] Compound 7 (6 g, 26.5 mmol) was dissolved in fuming nitric acid (60 mL) and stirred at room temperature for 16 hours. The reaction mixture was poured on ice, and the light yellow sludge formed was separated and then dissolved in ether to provide a solid. Filtration gave the title compound 8 as a white solid (1.15 g, 14%). 1 H NMR(DMSO-d 6 ) δ (ppm): 8.22 (d, J=9.0 Hz, 4H), 7.65 (d, J=8.6 Hz, 4H), 5.76 (s, 1H), 3.72 (s, 3H).
[0316] Step 2: N-hydroxy-2,2-bis(4-nitrophenyl)acetamide (9)
[0317] Following the same procedure as described in step 2 of Example 1, but replacing compound 2 with compound 8, the title compound 9 was obtained in 25% yield (50 mg). 1 H NMR(CD 3 OD-d 4 ) δ (ppm): 8.21 (d, J=8.8 Hz, 4H), 7.61 (d, J=8.6 Hz, 4H), 5.07 (s, 1H). LRMS(ESI): calculated value 317.1, measured value 316.3(M-H)-.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com