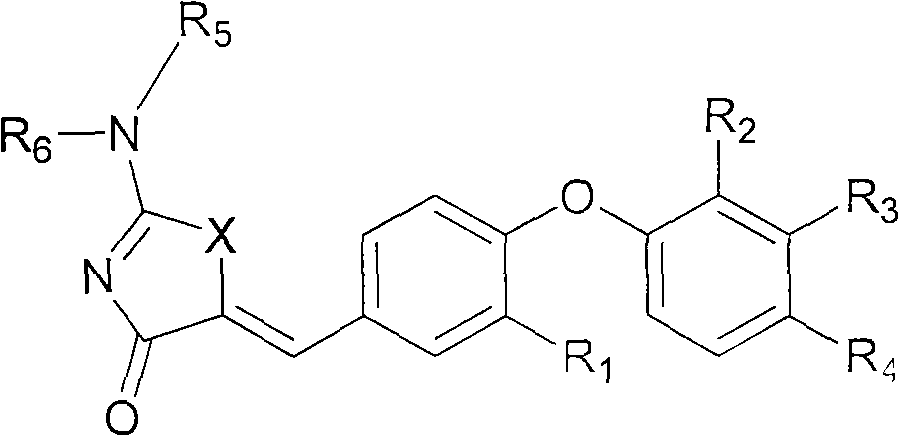
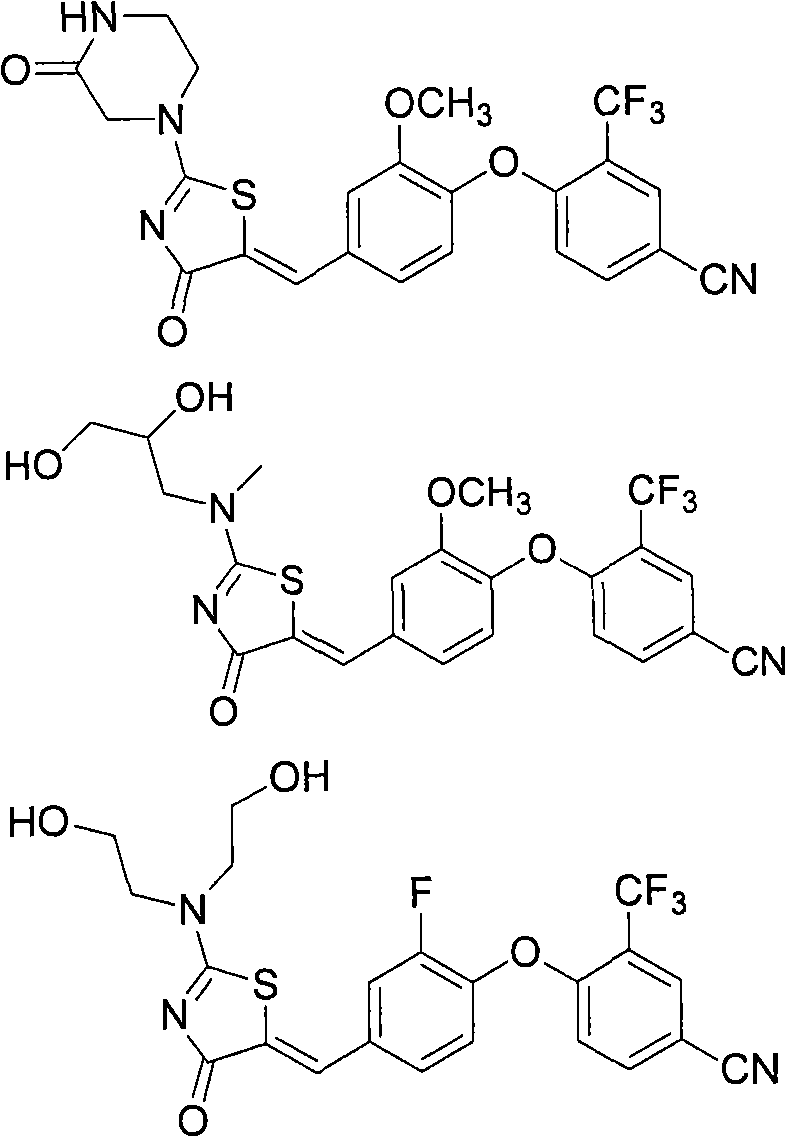
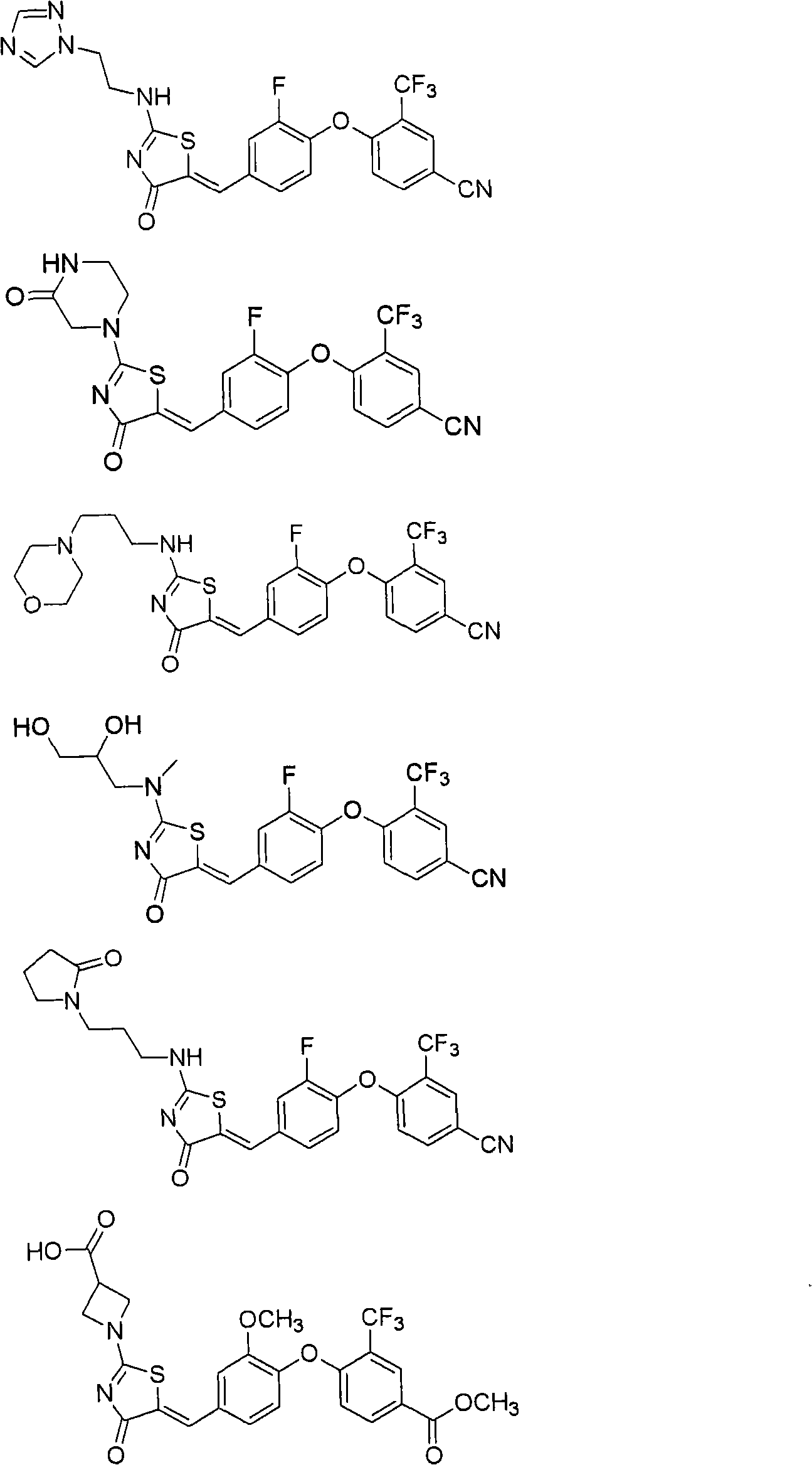
Substituted phenoxy aminothiazolones as estrogen related receptor-alpha modulators
An alkoxy and alkyl technology, applied in the field of substituted phenoxyaminothiazolones used as estrogen-related receptor-α modulators, can solve problems such as unknown molecular mechanisms
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0265] 4-[4-(2-Amino-4-oxo-4H-thiazol-5-ylidenemethyl)-2-methoxy-phenoxy]-3-trifluoromethyl-benzonitrile
[0266]
[0267] A. Preparation of 4-(4-formyl-2-methoxy-phenoxy)-3-trifluoromethyl- Benzonitrile. 1 H NMR (400Hz, CDCl 3 ) 10.00(s, 1H), 8.00(m, 1H), 7.68(dd, 1H), 7.58-7.53(m, 2H), 7.29(d, 1H), 6.75(d, 1H), 3.83(s, 3H) ;LC / MS(m / z)[M+1] + 322.1(C 16 h 11 f 3 NO 3 The calculated value of , 322.06).
[0268] B. Preparation of 4-[ 4-(2-Amino-4-oxo-4H-thiazol-5-ylidenemethyl)-2-methoxy-phenoxy]-3-trifluoromethyl-benzonitrile. 1 H NMR (400Hz, DMSO-d6) 8.96 (bs, NH), 8.74 (bs, NH), 8.32 (d, 1H), 7.99 (dd, 1H), 7.65 (d, 1H), 7.47 (d, 1H) , 7.36(d, 1H), 7.24(d, 1H), 6.89(d, 1H), 3.88(s, 3H); LC / MS(m / z)[M+1] + 420.0 (C 19 h 13 f 3 N 3 o 3 S, 420.38).
Embodiment 2
[0270] 4-[4-(2-Amino-4-oxo-4H-thiazol-5-ylidenemethyl)-2-methoxy-phenoxy]-3-chloro-benzonitrile
[0271]
[0272] A. Using general procedure A, 4-(2-chloro-4-cyanophenoxy)-3-methoxybenzaldehyde was prepared from vanillin and 3-chloro-4-fluorobenzonitrile. 1 H NMR (400Hz, CDCl 3 )δ9.95(s, 1H), 7.76(d, 1H), 7.56(br s, 1H), 7.50(d, 1H), 7.45(br d, 1H), 7.15(d, 1H), 6.78(d , 1H), 3.90 (s, 3H).
[0273] B. Preparation of 4-[4-(2- Amino-4-oxo-4H-thiazol-5-ylidenemethyl)-2-methoxy-phenoxy]-3-chloro-benzonitrile. 1 H NMR (400Hz, DMSO-d6) δ9.47 (bs, NH), 9.21 (bs, NH), 8.17 (d, 1H), 7.70 (dd, 1H), 7.64 (s, 1H), 7.46 (d, 1H), 7.32(d, 1H), 7.23(dd, 1H), 6.81(d, 1H), 3.79(s, 3H); LC / MS(m / z)[M+1] + 386.0 (C 18 h 13 ClN 3 o 3 Calculated value of S, 386.82).
Embodiment 3
[0275] 4-[2-Chloro-4-(2-imino-4-oxo-thiazolidin-5-ylidenemethyl)-phenoxy]-3-trifluoromethyl-benzonitrile
[0276]
[0277] A. Preparation of 4-[2-chloro-4-(2,4-dioxo-thiazole) from 3-chloro-4-hydroxybenzaldehyde and 4-fluoro-3-trifluoromethylbenzonitrile using general method A Alk-5-ylidenemethyl)-phenoxy]-3-trifluoromethylbenzonitrile. 1 H NMR (400MHz, CDCl 3 )δ9.99(s, 1H), 8.06(d, J=1.96Hz), 8.02(d, J=1.96Hz, 1H), 7.87(dd, J=8.22 and 1.96Hz, 1H), 7.76(dd, J=8.61 and 1.96 Hz, 1H), 7.27 (d, J=8.61 Hz, 1H), 6.82 (d, J=8.61 Hz, 1H).
[0278] B. According to general procedure C, using 2-amino-thiazol-4-one and 4-[2-chloro-4-(2,4-dioxo-thiazolidine-5-ylidenemethyl)-phenoxy ]-3-trifluoromethylbenzonitrile to prepare 4-[2-chloro-4-(2-imino-4-oxo-thiazolidine-5-ylidenemethyl)-phenoxy]-3-tri Fluoromethyl-benzonitrile. 1 HNMR (400MHz, DMSO-d6) δ 9.56 (br, 1H), 9.24 (br, 1H), 8.40 (d, J=1.56Hz, 1H), 8.08 (dd, J=8.6 and 1.95Hz, 1H), 7.90(d, J=1.96Hz, 1H), 7.65(d, J=2.35Hz, 1H), ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com