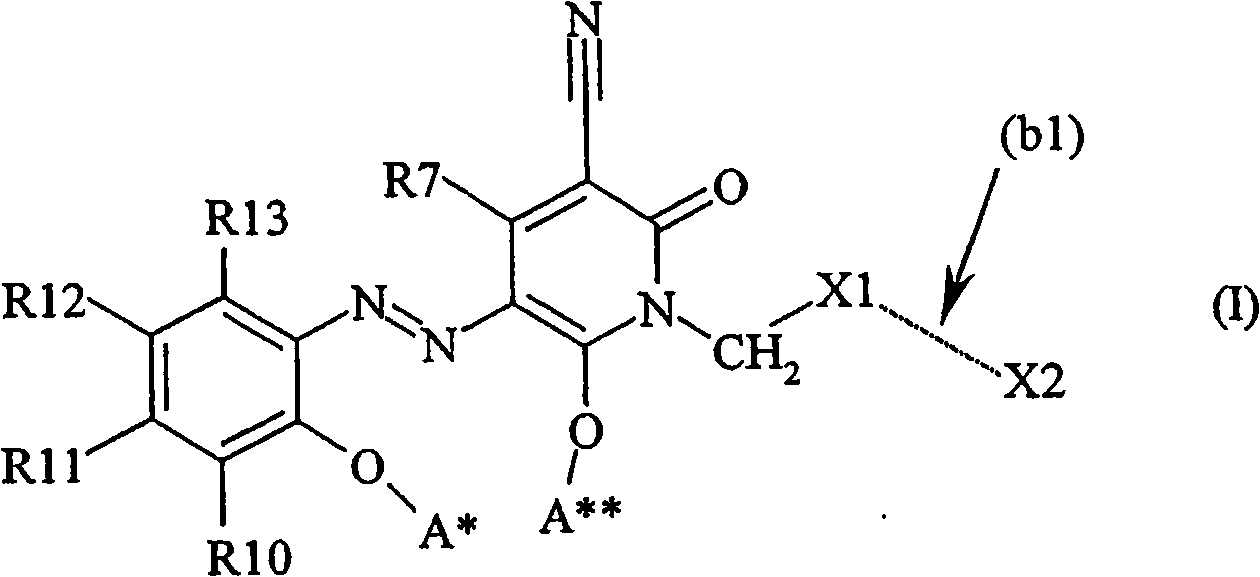
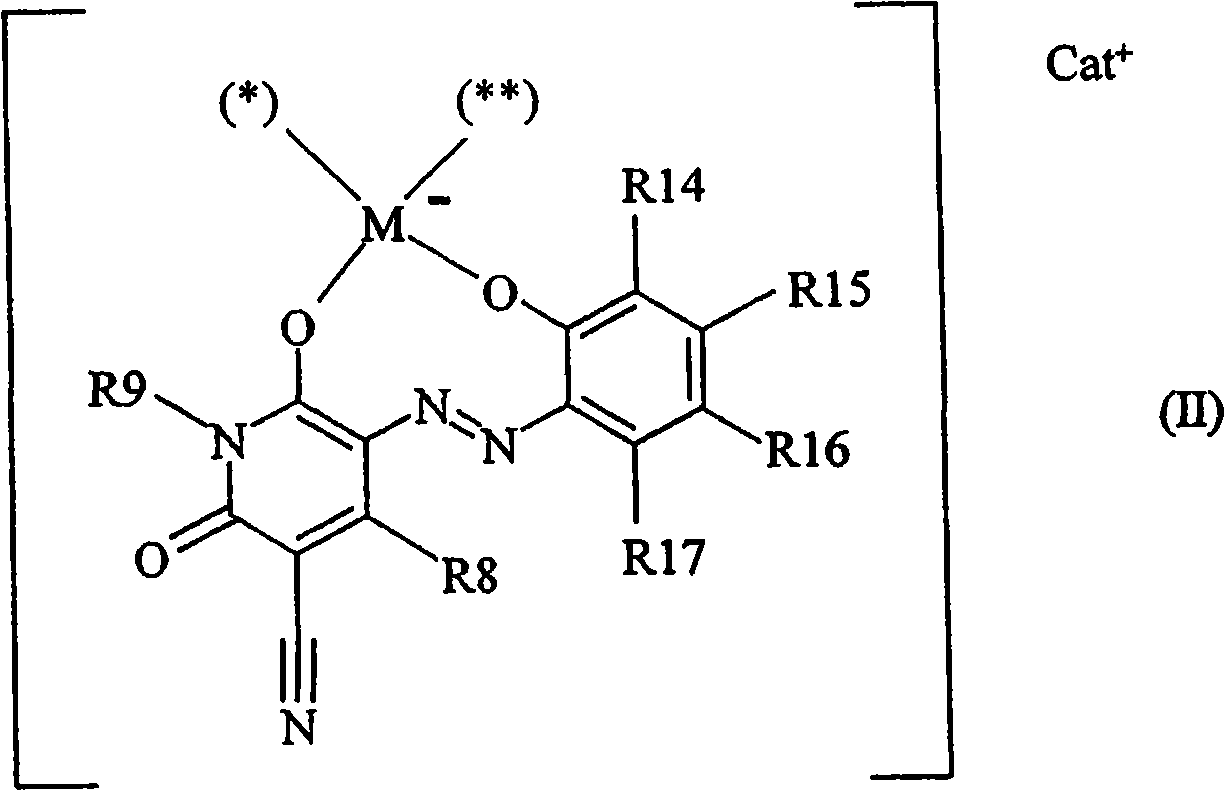
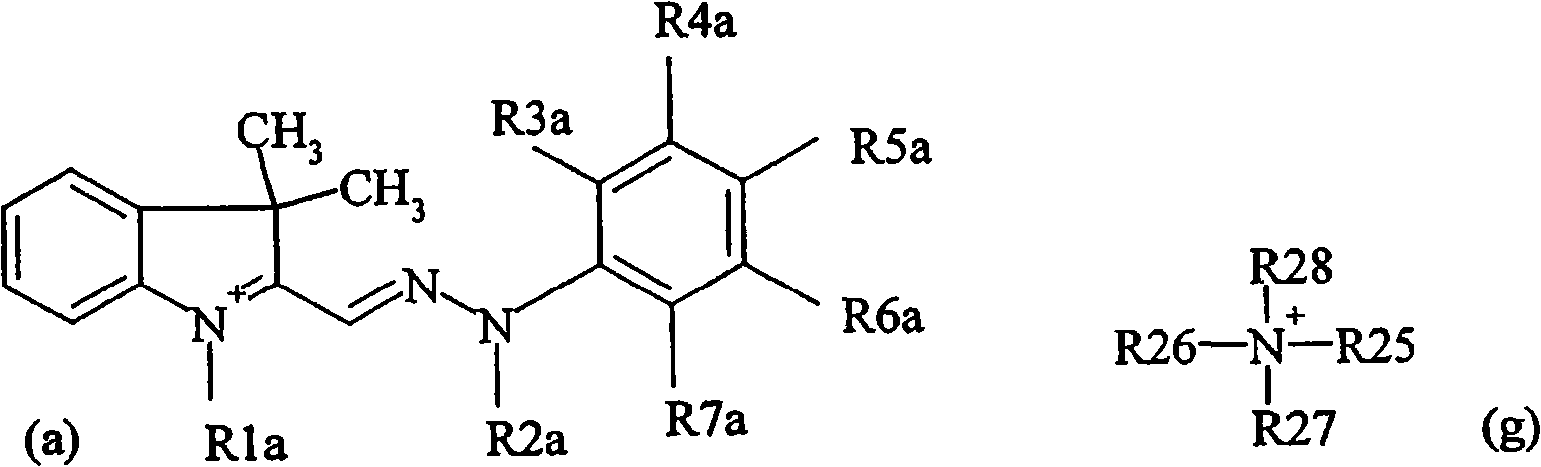
Pyridinone based azo dyes and their metal complex salts
A compound and metal atom technology, applied in the field of azo, can solve problems such as hindering dyes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0414]
[0415] 34.65 g of ethyl cyanoacetate was added dropwise to 17.48 g of allylamine. The resulting mixture was refluxed for 1 hour. The temperature was cooled to 70°C and 43.38 g ethyl acetoacetate was added dropwise followed by 48 g piperidine and 166 ml toluene respectively. The resulting mixture was refluxed overnight. Toluene was distilled off, 160 ml of water were added and the mixture was allowed to cool to room temperature.
[0416] The resulting slurry was transferred dropwise into 150 ml of cold methanol (ice bath). During the transfer, the pH was maintained at pH 1 by addition of concentrated aqueous HCl. The resulting slightly yellowish precipitate was filtered, washed 5 times with 200 ml each of water, and dried under vacuum at 60° C. for 24 hours. 37.7 g of the compound of formula (c1) are obtained as a white solid.
Embodiment 2
[0418]
[0419] 50 g of propargylamine were added to 51.36 g of ethyl cyanoacetate at room temperature under nitrogen over 20 minutes. The resulting mixture was stirred at room temperature under nitrogen for 12 hours. The precipitate was filtered, washed with toluene (75ml), and dried under vacuum at 80°C to give 48.57g of 2-cyano-N-prop-2-ynyl-acetamide.
[0420]51.79 g of ethyl acetoacetate were dissolved in ethanol (160 ml) at room temperature under nitrogen. The solution was cooled to 0°C and 27.08 g of sodium ethoxide was added. The resulting mixture was stirred for 10 min and 48.57 g of 2-cyano-N-prop-2-ynyl-acetamide were added portionwise. The suspension was refluxed for 15 hours while exchanging ethanol three times to remove water formed during the reaction. After cooling to room temperature, the mixture was diluted with ethanol, the solid was filtered and dried under vacuum at 80 °C to give the compound of formula (c2).
Embodiment 3
[0422]
[0423] The preparation according to Example 1 was carried out using cyanoacetate and acetoacetate in a stoichiometric ratio relative to the respective amine compound to obtain the compound of formula (c3).
[0424] Combinations and details are given in Table (A1).
[0425]
[0426] Table A2 shows the physico-chemical properties of the compounds of formulas (c1) to (c3).
[0427]
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com