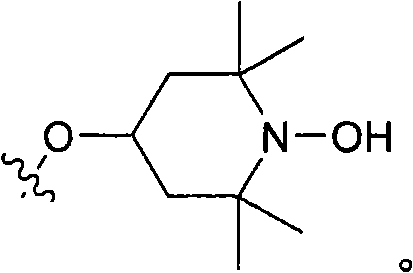
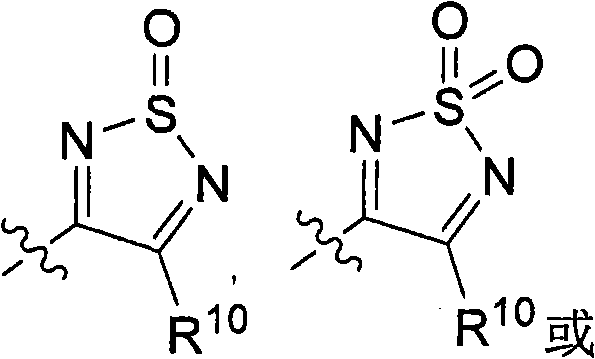
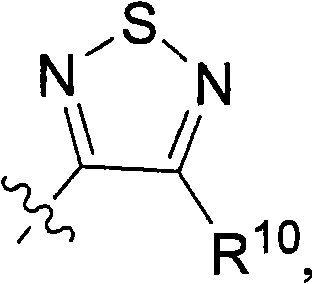
Hydroxylamine compounds and methods of their use
A compound, hydroxyl technology, applied in the field of new hydroxylamine compounds, can solve the problems of no reported anti-angiogenic activity of hydroxylamines, difficulty in production, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0388] Compound preparation
[0389] General method A: For the synthesis of 3-hydroxy-4-alkyl-1,2,5-thiadiazole or 3-chloro-4-alkyl-1,2,5-thiadiazole or 3-chloro-4 - General procedure for aryl-1,2,5-thiadiazoles
[0390] Aminoamide hydrochloride (20 mmol) was dissolved in 20 ml of DMF. Sulfur monochloride (7.6 g, 56 mmol) was added at 0-5°C over 20 minutes and the reaction was stirred at room temperature for 6 hours. The yellow sulfur precipitate was filtered after quenching the reaction with ice water. CH for aqueous phase 2 Cl 2 Extract (3x20ml). Combined organic phases with MgSO 4 dry. The solvent was removed and the residue was purified on a silica gel column using CH 2 Cl 2 (1 L) as eluent. 4-(2-Methyl-alkyl)-3-hydroxy-1,2,5-thiadiazole was obtained as a pale yellow crystalline solid.
[0391] When amino-nitriles are used instead of amino-amides, 3-chloro-4-alkyl-1,2,5-thiadiazoles or 3-chloro-4-aryl-1,2,5-thiadiazoles are obtained. pass 1 H NMR confirmed the...
Embodiment 1
[0395] General method for the preparation of nitrogen oxides:
[0396] To a solution of t-BuOK in t-BuOH (30 mL) was added TEMPOL in one portion, followed by thiadiazole. The reaction mixture was stirred overnight at room temperature. Water (50 mL) was then added to the reaction mixture. The mixture was extracted with EtOAc (3 x 50 mL). The organic phase was washed with brine (20 mL) and washed with MgSO 4 dry. Solvent was removed in vacuo. The crude product was purified by silica gel column (EtOAc / hexane=1 / 10). After removing the solvent in vacuo, the product (3.0 g) was obtained.
Embodiment 2
[0398] General procedure for the preparation of hydroxylamine HCl salt:
[0399] To a solution of nitrogen oxide compound (~1 g, 5.4 mmol) in 2-propanol (~10 mL) was added in one portion a saturated solution of hydrogen chloride in 2-propanol (~20 mL), and the reaction mixture was stirred at room temperature (1 h to 20 h) or heated to reflux (~2 h) until it became colorless. The solvent was removed in vacuo to give an off-white solid. The crude product was recrystallized from 2-propanol. A white solid (-0.72 g, 3.2 mmol) was obtained. pass 1 The product was identified by H NMR analysis, elemental analysis, IR and mp.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com