Process for the preparation of alfuzosin and salts thereof
A technology of alfuzosin and process, applied in the field of N-[3-[methylamino]propyl]tetrahydro-2-furancarboxamide compounds, can solve problems such as patient injury, and achieve the effect of low toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
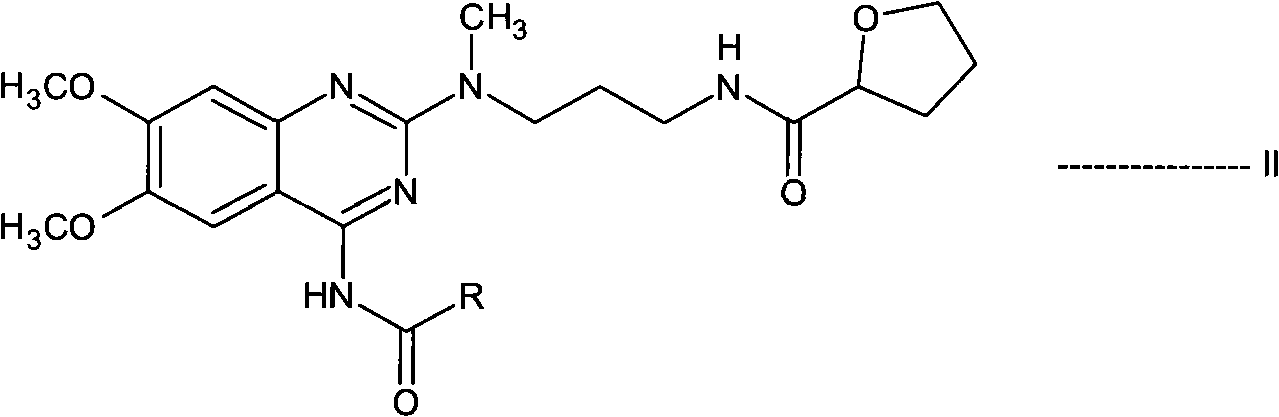
[0110] N-[3-[(4-acetylamino-6,7-dimethoxy-2-quinazolinyl)methylamino]propyl]tetrahydro-2-furancarboxamide (N-acetylalfurazole oxazine) preparation
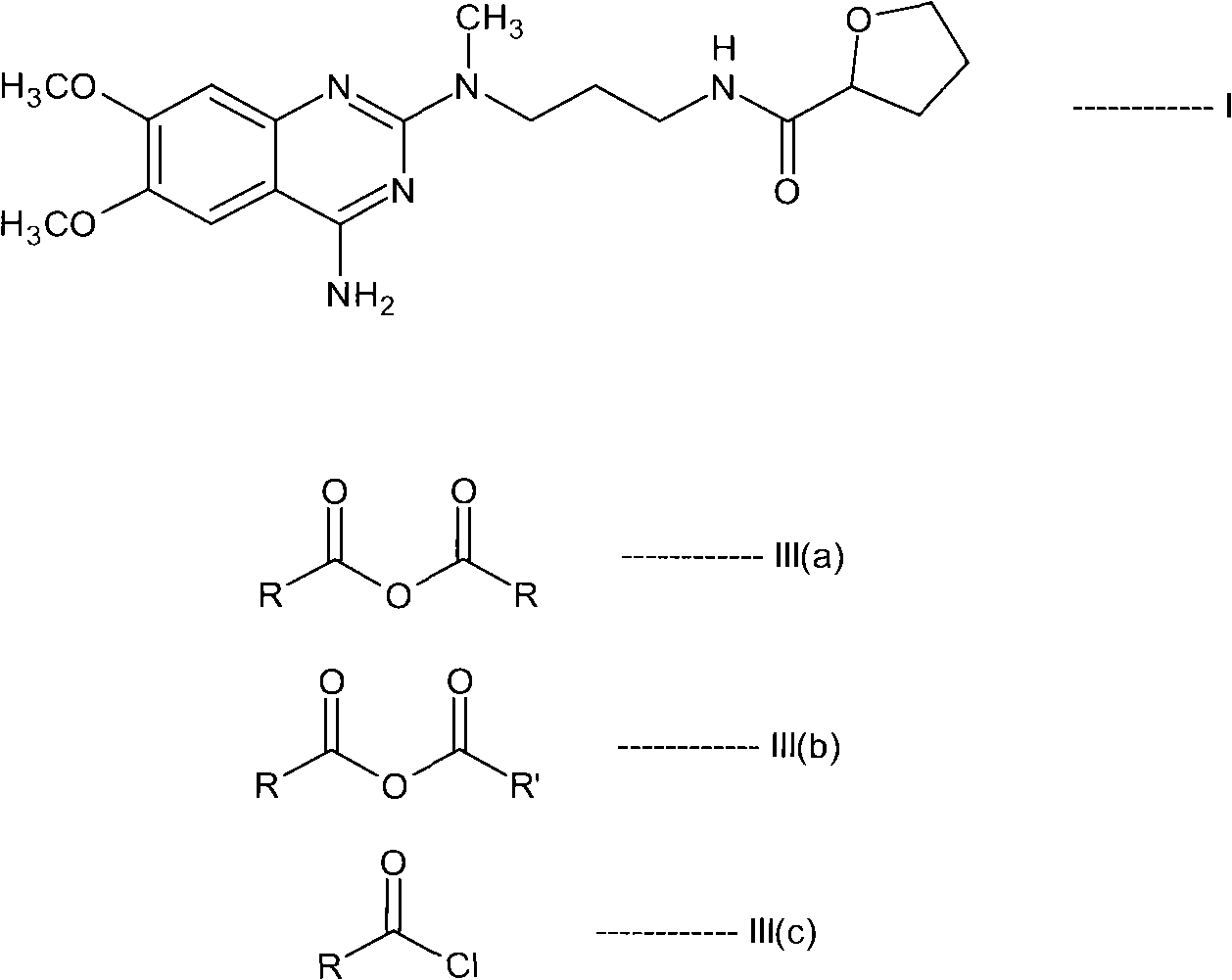
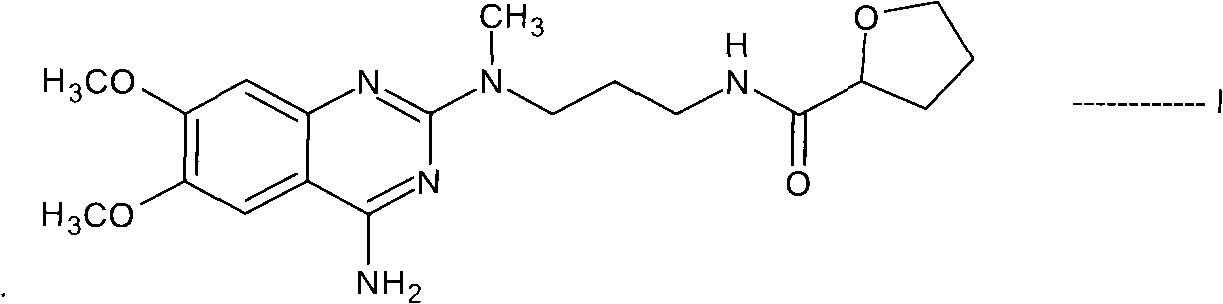
[0111] N-(4-amino-6,7-dimethoxyquinazolin-2-yl)-N-methyl-2-cyanoethylamine hydrochloride (55gm), saturated ammonia methanol solution (550 ml) and Raney nickel (82.5gm) mixture into a pressure vessel and hydrogenated under a pressure of 10kg. The reaction mass was heated to 80°C for 10 hours. The resulting reaction mass was cooled to 40°C, the catalyst was filtered off and washed with methanol (506 mL). The filtrate was distilled to obtain N-(4-amino-6,7-dimethoxyquinazolin-2-yl)-N-methylpropylenediamine. In dichloromethane (755ml), in the presence of N,N-carbonyldiimidazole (30.8gm), this diamine compound was reacted with tetrahydro-2-furoic acid (18.2ml) at 40°C for 4 hours to prepare Reaction mass containing N-[3-[(4-amino-6,7-dimethoxy-2-quinazolinyl)methylamino]propyl]tetrahydro-2-furancarboxamide. The reaction mass was c...
Embodiment 2
[0113] Preparation of Alfuzosin Hydrochloride
[0114] N-acetyl alfuzosin (40.0 gm) was dissolved in methanol (120 mL). The resulting solution was acidified with methanolic hydrochloride (27.68 mL). The reaction mixture was heated to 40 °C for 8 hours. The reaction mass was then cooled at 25°C. The isolated solid was filtered under nitrogen atmosphere, washed with methanol (75 mL), then dried in vacuo at 80-85 °C to prepare the title compound (yield: 90%; HPLC purity = 99.90%).
Embodiment 3
[0116] Preparation of Alfuzosin Hydrochloride
[0117] N-Acetyl alfuzosin (40.0 gm) was dissolved in isoamyl alcohol (207.66 mL). The resulting solution was acidified with methanolic hydrochloride (52.8 mL). The reaction mixture was heated to 40 °C for 16 hours. The reaction mass was then cooled at 25°C. The solid separated was filtered under nitrogen and washed with isoamyl alcohol (197.7 mL). The resulting wet cake was refluxed with ethyl acetate (280.48 mL) at 78°C for 30 minutes. The resulting solid was filtered and washed with ethyl acetate (117.51 mL), and then dried in vacuo at 110° C. to prepare the title compound (yield: 90%; HPLC purity: 99.93%; N-acetyl alfuzosin impurity content : 0.04%). The isolated anhydrous alfuzosin hydrochloride was confirmed to be polymorphic Form I.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com