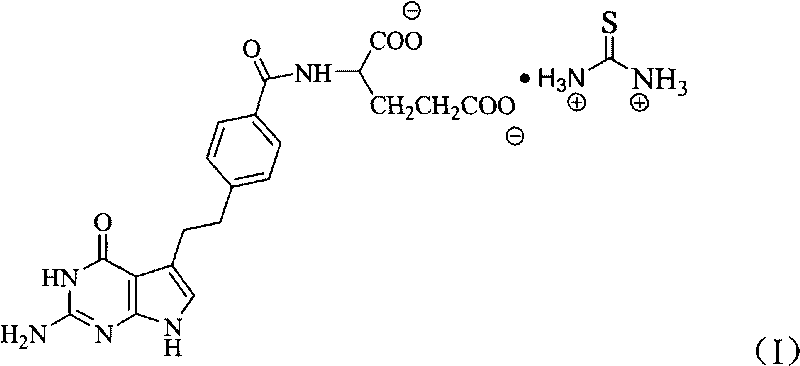
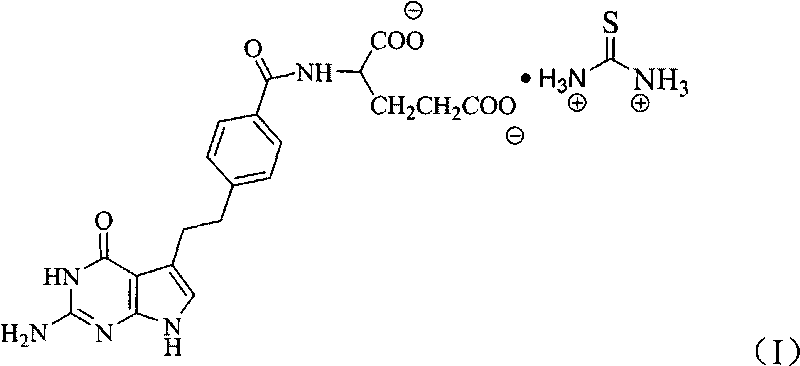
Pemetrexed thiocarbamide salt and preparation method thereof
A technology of pemetrexed thiourea salt and pemetrexed, which is applied in the field of pharmacy, can solve the problems of complex preparation process, poor stability, and low yield, and achieve the effect of easy preparation and good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Embodiment 1 Preparation process 1 of pemetrexed thiourea salt
[0023] In a 100ml reaction bottle, put 2.50g (5.85mmol) of pemetrexed into it, add 25ml of deionized water, 0.668g (8.78mmol) of thiourea salt, stir and react at room temperature for 1 hour, filter, and the filtrate is distilled under reduced pressure to precipitate a solid, add Methanol, until most of the product was precipitated, filtered, and vacuum-dried to obtain 2.26g, yield 76.4%.
[0024] Elemental analysis: C: 49.90 (49.79% of theoretical value), H: 5.49 (5.57% of theoretical value), N: 19.53 (19.36% of theoretical value).
[0025] Infrared spectrum (KBr tablet): characteristic absorption peak (cm -1 ) and its attribution: 3330 (C-NH 2 , -NH, -OH), 3046 (C 6 h 4 , benzene ring hydrocarbon stretching vibration), 2929 (-CH 2 -), 2510(-NH 3 '), 1648 (C=O), 1500, 1392 (C 6 h 4 , benzene ring skeleton vibration).
[0026] 1 H NMR spectrum (DMSO-d 6 ), chemical shift δ (ppm) and assignment o...
Embodiment 2
[0027] Embodiment 2 The preparation process 2 of pemetrexed thiourea salt
[0028] In a 100ml reaction bottle, put 2.50g (5.85mmol) of pemetrexed, add 60ml of anhydrous methanol, stir to dissolve, add 0.445g (5.85mmol) of thiourea, stir and react at room temperature for 0.5 hours, filter, wash with methanol and water , dried in vacuum to obtain 1.64g, yield 55.3%.
Embodiment 3
[0029] Embodiment 3 The preparation process 3 of pemetrexed thiourea salt
[0030] In a 100ml reaction bottle, put 2.50g (5.85mmol) of pemetrexed, add 60ml of absolute ethanol, stir to dissolve, add 2.23g (29.27mmol) of thiourea, stir at room temperature for 1 hour, filter, wash with ethanol and water , dried in vacuo to obtain 1.90 g, yield 64.2%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com