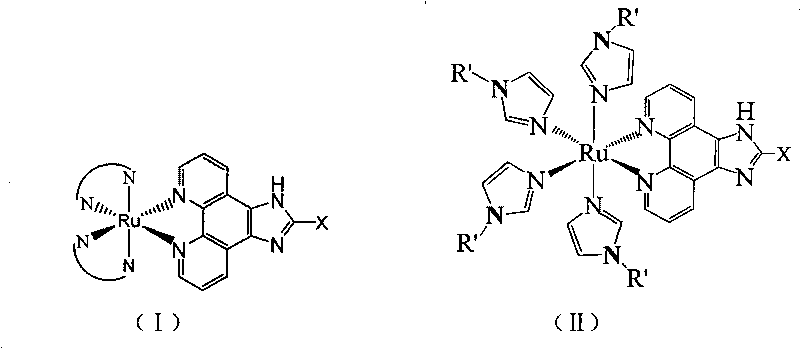
Ruthenium complex, preparation method thereof and application thereof
A technology of ruthenium complexes and compositions, applied in the field of ruthenium complexes with anti-tumor activity and their preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
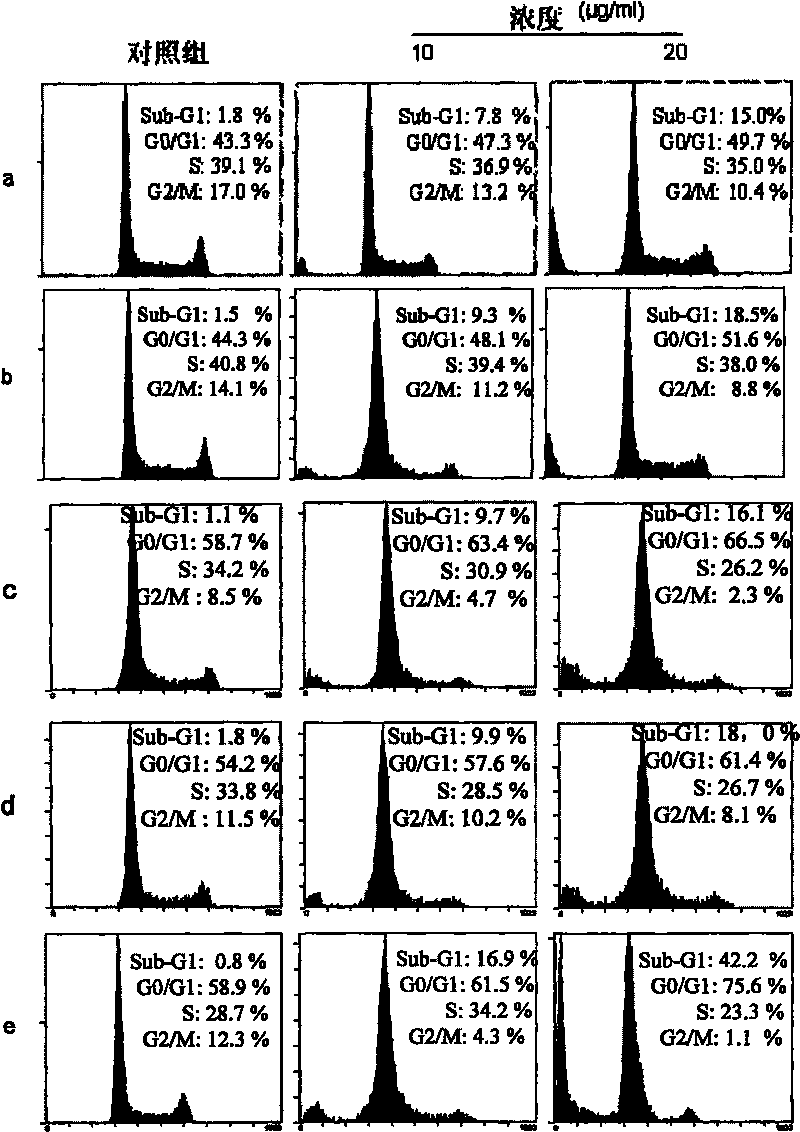
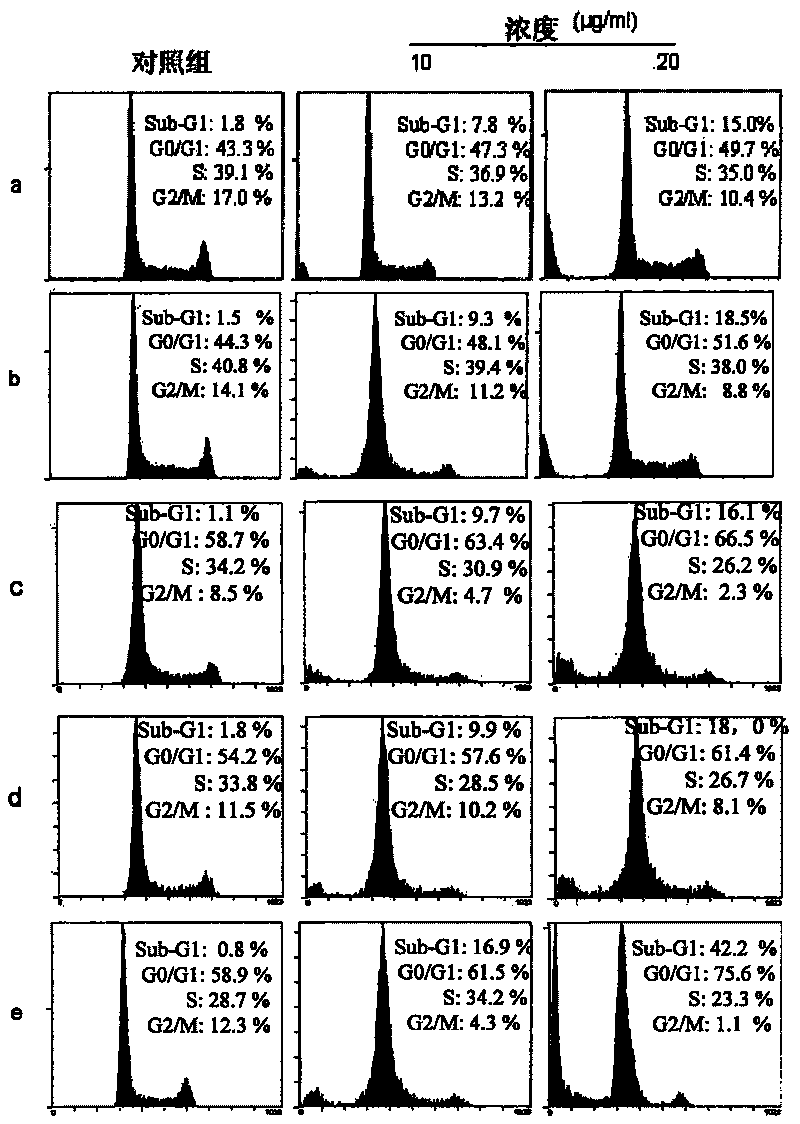
Image
Examples
Embodiment 1
[0089] Embodiment 1 invention described complex a: Ru(bpy) 2 Preparation of p-MOPIP
[0090] (1) Synthesis of phenanthroline 5,6-dione
[0091] Mix 4.0g o-phenanthroline and 4.0g potassium bromide and place in a round bottom flask, then add cold concentrated H 2 SO 4 (40mL) and concentrated HNO 3 (20mL) of mixed acid. After adding the mixed acid dropwise, keep the temperature at 80-85°C for 3 hours, cool down, pour it into 500mL ice water, and then use 10mol L -1 The NaOH solution was carefully neutralized to neutral. The solution was extracted with 3 x 100 mL of chloroform. After combining the organic phases, they were washed with 50 mL of water and finally dried overnight with sodium sulfate. After filtration, the chloroform was distilled off under reduced pressure, and the solid product was recrystallized from ethanol to obtain yellow or orange needle-like crystals. Yield: 75%.
[0092] (2) Synthesis of 2-(4-methoxyphenyl)-1H-imidazo[4,5-f][1,10]phenanthroline (p-m...
Embodiment 2
[0098] Embodiment 2 invention said complex b: Ru(phen) 2 Preparation of p-MOPIP
[0099] (1) Synthesis of 2-(4-methoxyphenyl)-1H-imidazo[4,5-f][1,10]phenanthroline (p-mopip): see the steps in the preparation of complex a (2).
[0100] (2)cis-[Ru(phen) 2 Cl 2 ]·3H 2 o
[0101] called RuCl 3 ·nH 2 O 1.56g (about 6mmol), with o-phenanthroline 2.40g (12mmol) and lithium chloride 1.68g (28mmol), dissolve it in 10mL DMF, and heat to reflux for 8h under the protection of argon. After cooling to room temperature, add 50 mL of acetone and freeze overnight. After suction filtration, the precipitate was washed with ice water and cold acetone, and dried in vacuum to obtain purple-black microcrystals. Yield, 72%.
[0102] (3)[Ru(phen) 2 p-MOPIP] 2+ Synthesis
[0103] [Ru(phen) 2 Cl 2 ]·2H 2 O (0.090g, 0.17mmol), synthesis of 2-(4-methoxyphenyl)-1H-imidazo[4,5-f][1,10]phenanthroline (p-mopip) (0.058g, 0.17mmol) and ethylene glycol (10mL) were refluxed for 2h under the prote...
Embodiment 3
[0104] Example 3 Invention of the complex c: Ru(Meim) 4 Preparation of p-MOIP
[0105] (1) Synthesis of 2-(4-methoxyphenyl)-1H-imidazo[4,5-f][1,10]phenanthroline (p-mopip): see the steps in the preparation of complex a (2).
[0106] (2)[Ru(p-mopip)Cl 4 ]Synthesis
[0107] 0.14g RuCl 3 ·xH 2 O and 0.163g aip were added to 30mL, 1M HCl, and stirred for 30 minutes under the protection of argon to dissolve it. Seal the reaction system and let it stand for 10 days under the protection of argon, filter it with suction, and wash it with water until it becomes colorless to obtain a dark green solid. Yield: 55%.
[0108] (3)[Ru(MeIm) 4 (4mopip)] (ClO 4 ) 2 2H 2 Synthesis of O
[0109] [Ru(p-mopip)Cl 4 ] (0.142g, 0.25mmol) and 1-methylimidazole (0.41g, 5mmol) were added to DMF (N,N-dimethylformamide) (15mL), stirred and refluxed for 8h under the protection of argon to obtain a dark red solution. Cool to room temperature, filter, rotary evaporate to 5 mL, then add 10 mL of ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com