Galanthamine long-acting release injectable microsphere composite and preparation method thereof
A technology of galantamine and galantamine base, which is applied in the direction of drug combination, active ingredients of heterocyclic compounds, medical preparations of non-active ingredients, etc., and can solve problems such as the effect of acupuncture
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
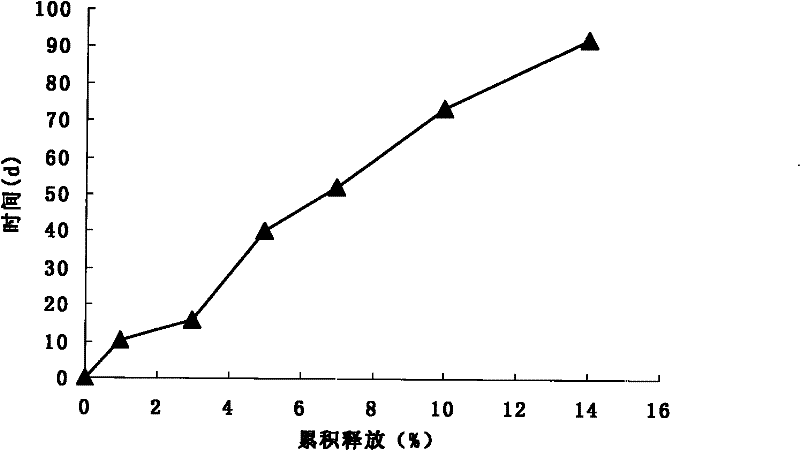
[0038] Embodiment 1: Galantamine PLGA microspheres were prepared by a vibrating nozzle method.
[0039] Precisely weigh about 55 mg of galantamine base, about 1000 mg of PLGA (monomer ratio LA:GA 50:50, molecular weight 12000) and add it to 5 ml of dichloromethane, vortex to dissolve completely, and make the dispersed phase. The dispersed phase was transferred to a 20ml glass syringe. Microspheres were prepared by vibrating nozzle method combined with emulsification method (O / W)-solvent evaporation method. The preparation instrument adopts the improved vibrating nozzle method microsphere instrument, and the process parameters are as follows: the aperture of the nozzle is 50 μm, the vibration frequency is 2000 Hz, the electric field voltage is 500 V, and the flow rate of the dispersed phase liquid is 1 ml / min, so that the nozzle can produce uniform and continuous droplets. Drop into the continuous phase of 200ml of 5% PVA17-88 aqueous solution, stir with medium-speed magnetic ...
Embodiment 2
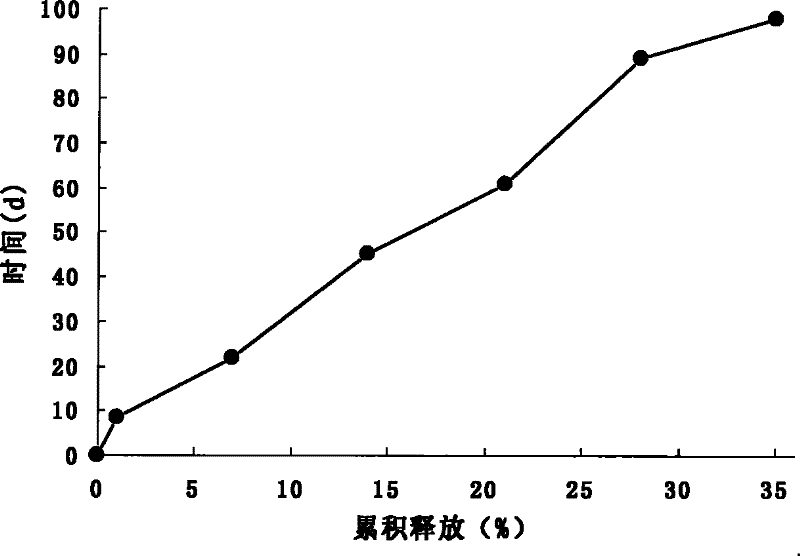
[0040] Example 2: Preparation of galantamine PLGA microspheres by vibrating nozzle method.
[0041] Precisely weigh about 130 mg of galantamine base, about 1000 mg of PLGA (monomer ratio LA:GA 50:50, molecular weight 12000) and add it to 20 ml of ethyl acetate, vortex to dissolve completely, and make the dispersed phase. The dispersed phase was transferred to a 20ml glass syringe. Microspheres were prepared by vibrating nozzle method combined with emulsification method (O / W)-solvent evaporation method. The preparation equipment adopts the improved vibrating nozzle method microsphere instrument, and the process parameters are as follows: the aperture of the nozzle is 25 μm, the vibration frequency is 3000 Hz, the electric field voltage is 1000 V, and the flow rate of the dispersed phase liquid is 2 ml / min, so that the nozzle can produce uniform and continuous droplets , drop into the continuous phase of 200ml of 2% PVA17-50 aqueous solution, stir magnetically at a medium speed...
Embodiment 3
[0042] Embodiment 3: Preparation of galantamine PLGA microspheres by vibrating nozzle method.
[0043] Precisely weigh about 300mg of galantamine base, about 1000mg of PLGA (monomer ratio LA:GA 50:50, molecular weight 12000) and add it to 10ml of dichloromethane, vortex to dissolve completely, and make the dispersed phase. The dispersed phase was transferred to a 20ml glass syringe. Microspheres were prepared by vibrating nozzle method combined with emulsification method (O / W)-solvent evaporation method. The process parameters are as follows: the aperture of the nozzle is 50 μm, the vibration frequency is 2000 Hz, the electric field voltage is 1500 V, and the flow rate of the dispersed phase liquid is 1 ml / min, so that the nozzle can produce uniform and continuous droplets. In the phase, magnetically stir at a medium speed for 1 hour, then magnetically stir at a low speed for 4 hours, reduce pressure to promote the development of the organic solvent, collect the microspheres ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com