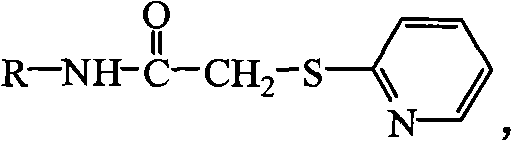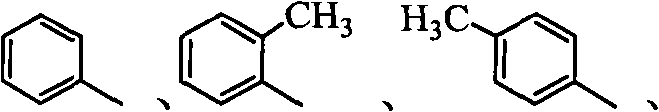Aromatic sulfur acetyl pyridine derivative and preparation method and application thereof
A technology for pyridylthioacetylaminoaromatics and derivatives, which is applied in the field of pesticide bactericidal preparations, pyridylthioacetylaminoaromatic derivatives and their preparations, and can solve the problems that the application of pyridylthioacetylaminoaromatic derivatives has not been reported, and achieve good results. Protective and therapeutic effects, easy control of reaction conditions, and simple preparation methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Example 1: 2-(pyridine-2-sulfur)-N-o-methylphenylacetamide (abbreviated as L 1 )Synthesis
[0020]
[0021] Add 0.26g (2mmol) of 2-mercaptopyridine and 5mL of ethanol (or a mixture of methanol or methanol and ethanol, the amount of which can completely dissolve 2-mercaptopyridine) into a 25mL single-necked round bottom flask, Dissolve at 30-60°C for half an hour until completely dissolved (as long as the 2-mercaptopyridine is completely dissolved, KOH or NaOH can be added for activation, not limited to half an hour or more), when the temperature is maintained at 30-50 At ℃, add 0.13g (2.4mmol) of KOH (or add 2.4mmol NaOH) to fully activate the 2-mercaptopyridine, then add 0.56g (3.2mmol) o-methyl chloride acetanilide, thin layer chromatography (TLC) follow the reaction Process, react for 36 hours, after the reaction is finished, slowly pour the reaction solution into 20mL of water (the amount of water is usually too much), let it stand for half an hour, filter it wi...
Embodiment 2
[0024] Example 2: 2-(pyridine-2-sulfur)-N-phenylacetamide (abbreviated as L 2 )Synthesis
[0025]
[0026] The synthesis method and characterization method are the same as in Example 1, except that 2-mercaptopyridine is dissolved at 40°C, the ratio of KOH to 2-mercaptopyridine is 1.2, and the activation is carried out at 30°C. The reaction between chloroacetanilide and 2-mercaptopyridine is adopted, and the ratio of the substance amount of chloroacetanilide to 2-mercaptopyridine is 1.8. The reaction finally yielded a white solid with a yield of 67.4% and a melting point of 56-57°C.
[0027] IR (KBr) v: 3324, 1674, 1578, 1438, 1116, 744cm -1 ;
[0028] 1 H NMR (CDCl 3 ): δ10.126 (b, 1H, NHCO), 7.581-8.537 (m, 1H, 2-thiopyridine ring C 6 -H), 7.587-7.554 (3H, 2-thiopyridine ring C 4 -H, benzene ring C 2 -H,C 6 -H), 7.256-7.304 (3H, 2-thiopyridine ring C 3 -H, benzene ring C 3 -H,C 5 -H), 7.039-7.138 (2H, 2-thiopyridine ring C 5 -H, benzene ring C 4 -H), 3.859-4....
Embodiment 3
[0029] Example 3: 2-(pyridine-2-sulfur)-N-p-methylphenylacetamide (abbreviated as L 3 )Synthesis
[0030]
[0031] The synthesis method and characterization method are the same as in Example 1, except that 2-mercaptopyridine is dissolved at 60°C, the ratio of KOH to 2-mercaptopyridine is 1.5, and the activation is carried out at 50°C. The reaction between p-methylchloroacetanilide and 2-mercaptopyridine is adopted, and the ratio of the substance amount of p-methylchloroacetanilide to 2-mercaptopyridine is 2.0. The reaction finally yielded a white solid with a yield of 82.7% and a melting point of 130-131°C.
[0032] IR(KBr)υ: 3296, 1654, 1446, 1148, 816cm -1 ;
[0033] 1 H NMR (CDCl 3 ): δ9.55(1H, NHCO), 8.55(1H, 2thiopyridine ring C 6 -H), 7.61 (1H, pyridine ring C 4 -H), 7.38 (2H, benzene ring C 2 -H,C 6 -H), 7.34 (1H, pyridine ring C 3 -H), 7.28 (1H, pyridine ring C 5 -H), 7.15 (2H, benzene ring C 3 -H, C5-H), 3.95 (2H, CH 2 S), 2.30 (3H, CH 3 ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com