Synthesis method of chloroanthraquinone
A synthetic method, the technology of chloroanthracene, applied in the field of preparation of fine chemicals, can solve the problems of lowering the export price of products, difficult and troublesome metal mercury treatment, and achieve the effect of improving quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
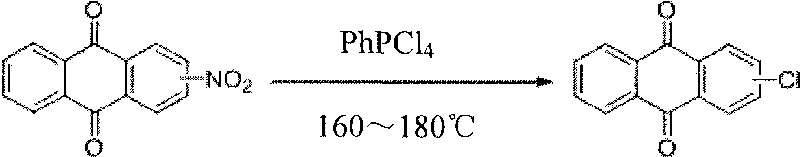
[0016] Preparation of 1-chloroanthraquinone
[0017] Pass 3.0g (42.3mM) of chlorine gas into the mixture of 4mL of dichlorophenylphosphine and 50mL of phenylphosphonic dichloride at room temperature, heat the reaction slightly, but do not exceed 30°C, previously colorless The transparent solution turned into a light yellow transparent solution, and 7.1 g (28.1 mM) of 1-nitroanthraquinone was added to the mixed solution, and then heated to 170° C. for 5 h. After cooling the reaction solution, the reaction solution was poured into 500 mL of water, then neutralized to pH 7 with 50% sodium hydroxide solution, and the neutralized solution was extracted with 500 mL (5×100 mL) of ethyl acetate, and the organic phase was mixed after extraction. , then use 200mL (2 × 100mL) saturated saline solution to elute the organic phase, dry with 5% anhydrous magnesium sulfate after the elution, then suction filter to obtain the filtrate, and obtain the concentrated solution after the filtrate is...
example 2
[0019] Preparation of 2-chloroanthraquinone
[0020] Pass 3.0g (42.3mM) of chlorine gas into the mixture of 4mL of dichlorophenylphosphine and 50mL of phenylphosphonic dichloride at room temperature, heat the reaction slightly, but do not exceed 30°C, previously colorless The transparent solution turned into a light yellow transparent solution, and 7.2 g (28.3 mM) of 2-nitroanthraquinone was added to the mixed solution, and then heated to 170° C. for 5 h. After cooling the reaction solution, the reaction solution was poured into 500 mL of water, then neutralized to pH 7 with 50% sodium hydroxide solution, and the neutralized solution was extracted with 500 mL (5×100 mL) of ethyl acetate, and the organic phase was mixed after extraction. , then use 200mL (2 × 100mL) saturated saline solution to elute the organic phase, dry with 5% anhydrous magnesium sulfate after the elution, then suction filter to obtain the filtrate, and obtain the concentrated solution after the filtrate is...
example 3
[0022] Preparation of 1,5-dichloroanthraquinone
[0023] Pass 12.2g (171.0mM) of chlorine gas into the mixture of 16mL of dichlorophenylphosphine and 200mL of phenylphosphonic dichloride at room temperature, heat the reaction slightly, but do not exceed 30°C, colorless before The transparent solution turned into a light yellow transparent solution, and 17.0 g (57.0 mM) of 1,5-dinitroanthraquinone was added to the mixed solution, and then heated to 170° C. for 5 h. After the reaction solution was cooled, the reaction solution was poured into 1000 mL of water, then neutralized to pH 7 with 50% sodium hydroxide solution, and the neutralized solution was extracted with 1000 mL (5×200 mL) of ethyl acetate, and the organic phase was mixed after extraction. , then elute the organic phase with 400mL (2×200mL) saturated saline solution, dry with 5% anhydrous magnesium sulfate after the elution, and then filter with suction to obtain the filtrate, and vacuum rotary distillation of the f...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com