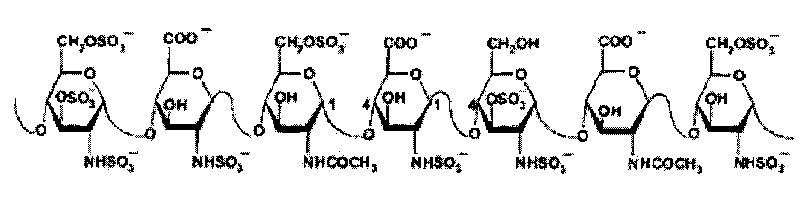
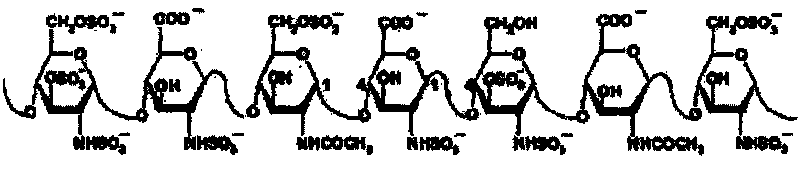
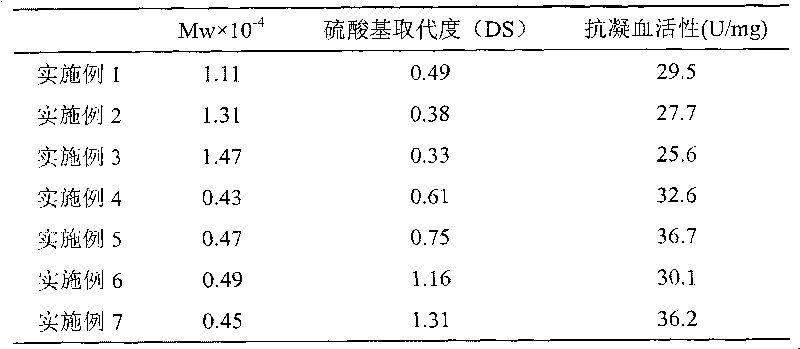
Method for preparing heparinoid polysaccharide
A technology of heparin and polysaccharide, applied in the field of medical polymers, can solve the problems of influence, heparin is too different, and the content of 2-position acetyl group is not considered.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Disperse 20g of chitin powder (commercially available chitin can be used, and in the embodiment of the present invention, chitin with 60 mesh and 9% degree of deacetylation) is dispersed in 200ml of phosphoric acid with a concentration of 85%, stirred, and the chitin is treated. After all the solution is dissolved, add this solution to 4000ml distilled water, stir rapidly while adding, precipitate white colloidal chitin, centrifuge to remove water, wash with distilled water until neutral, and obtain the acid-treated product. Disperse the acid-treated product in 1000ml of distilled water, stir in an ice-water bath, add 240mg Tempo and 8g NaBr to completely dissolve Tempo and NaBr, then add 480ml of 4% NaClO solution, and continuously add 0.5M NaOH solution dropwise to make the reaction During the process, the pH value of the solution was stabilized at 10.8, reacted for 1 hour, then added 50ml of ethanol, adjusted the pH value to neutral with 2M acetic acid, purified the s...
Embodiment 2
[0021]Disperse 5g of chitin powder (60 mesh, deacetylation degree 15%) into 90ml of 65% phosphoric acid, stir until the chitin is completely dissolved, add this solution to 300ml of distilled water, stir rapidly while adding, and precipitate a white gum After the chitin was formed, it was centrifuged to remove water, and the precipitate was washed with distilled water until neutral to obtain the acid-treated product. This acid treatment product is dispersed in 150ml distilled water, under ice-water bath condition, stir, add 75mg Tempo and 1.5g NaBr, Tempo and NaBr are completely dissolved, then add 40ml 10% NaClO solution, continue dripping the KOH solution of 2M to make During the reaction, the pH value of the solution was stabilized at 9.6, reacted for 0.5h, then added 10ml of methanol, adjusted the pH value to neutral with 4M HCl, purified the solution by ultrafiltration, concentrated by distillation under reduced pressure, precipitated with acetone, and dried in vacuo to ob...
Embodiment 3
[0024] Disperse 2g of chitin powder (60 mesh, deacetylation degree 15%) into 38ml of 70wt% phosphoric acid, stir until the chitin is completely dissolved, add this solution to 200ml of distilled water, stir rapidly while adding, and precipitate a white gum Centrifuge the chitin to remove water, wash with distilled water until neutral, and obtain the acid-treated product. Disperse the acid-treated product in 50ml of distilled water, stir in an ice-water bath, add 16mg of Tempo and 0.8g of NaBr to completely dissolve Tempo and NaBr, then add 16ml of 8% NaClO solution, and continue to drop 2M of NaBr 2 CO 3 Make the pH value of the solution during the reaction process 10.0, react for 0.5h, then add 4.5ml ethanol, adjust the pH value to neutral with 3M phosphoric acid, and then dialyze the solution against distilled water for 72h, distill and concentrate under reduced pressure, precipitate with anhydrous methanol, and dry in vacuo The 6-position selective oxidation reaction produ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com