Solid medicinal composition of clopidogrel hydrogen sulfate
A kind of technology of clopidogrel hydrogen sulfate and composition, applied in the stable oral pharmaceutical composition field containing I crystal form clopidogrel hydrogen sulfate, achieves the effects of good reproducibility, stable quality, and overcoming instability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
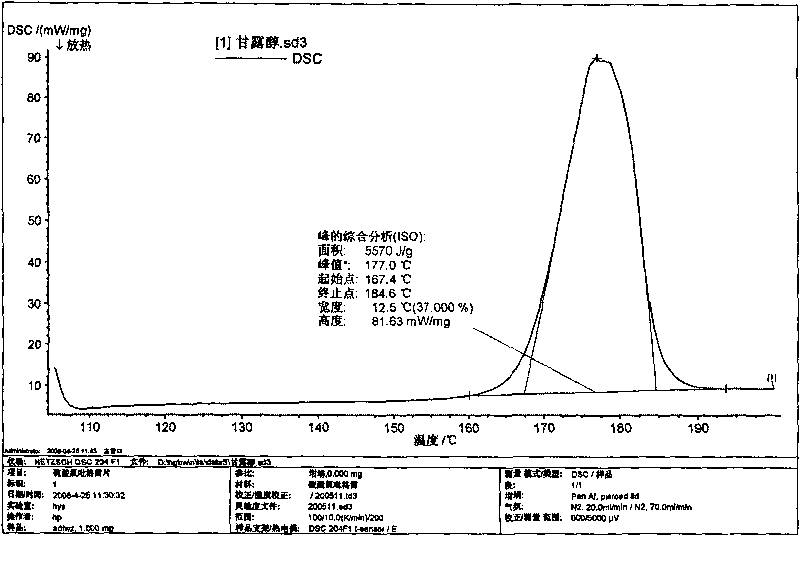
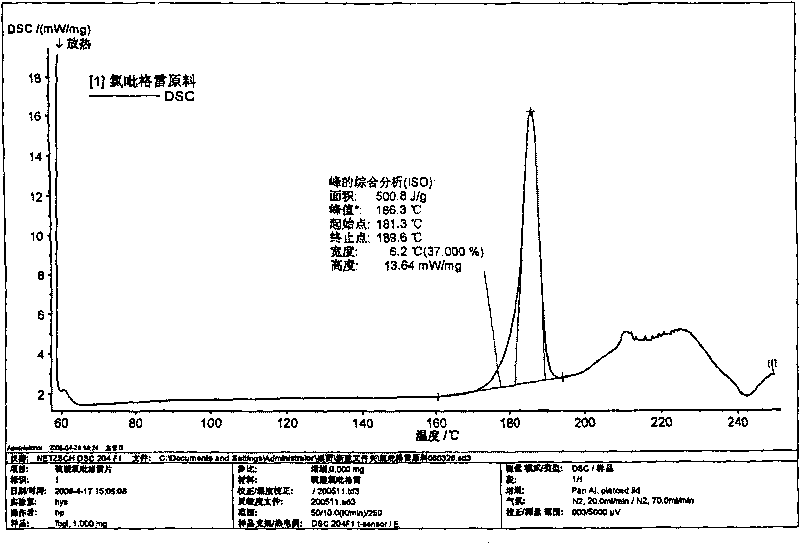
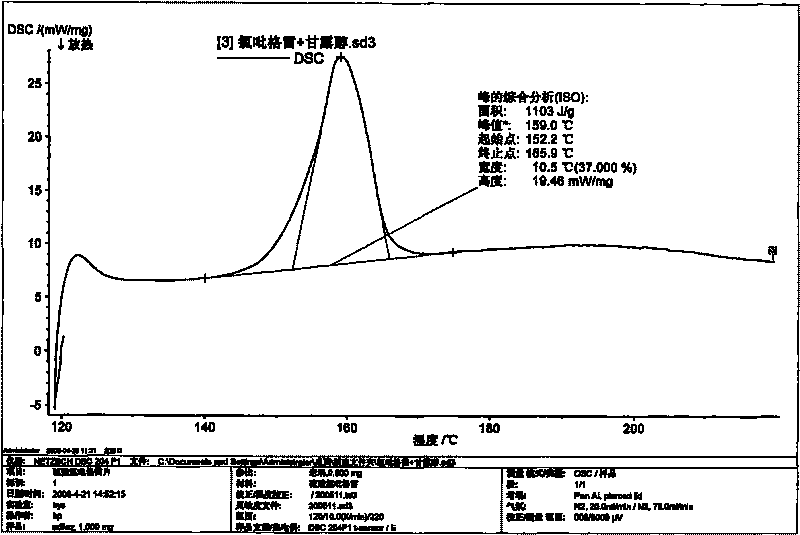
Image
Examples
Embodiment 1
[0040] Raw material name 1000 tablets dosage (g)
[0041] Form I Clopidogrel Bisulfate 97.9 (equivalent to Clopidogrel 75g)
[0042] PEG6000 3
[0043] Micronized silica gel 10
[0044] Microcrystalline Cellulose 135
[0045] Low-substituted hydroxypropyl cellulose 12.5
[0046] Gastric-soluble film coating premix appropriate amount
[0047] 1) Pass I crystalline form clopidogrel bisulfate through a 40-mesh sieve for later use.
[0048] 2) Pass the microcrystalline cellulose, low-substituted hydroxypropyl cellulose and micropowder silica gel through a 60-mesh sieve respectively, and dry at 105°C for 2 hours for later use.
[0049] 3) The polyethylene glycol 6000 is jet-pulverized into fine powder for later use.
[0050] 4) After mixing the prescription amount of crystalline form clopidogrel hydrogen sulfate, microcrystalline cellulose and low-substituted hydroxypropyl cellulose evenly, pass through a dry granulator, and granulate with a 20-mesh sieve.
[0051] 5) Pass t...
Embodiment 2
[0056] Raw material name 1000 tablets dosage (g)
[0057] Form I Clopidogrel Bisulfate 32.6 (equivalent to Clopidogrel 25g)
[0058] Polyethylene glycol 6000 150
[0059] Micronized silica gel 30
[0060] Pregelatinized starch 85
[0061] Low-substituted hydroxypropyl cellulose 22
[0062] Gastric-soluble film coating premix appropriate amount
[0063] 1) Pass I crystalline form clopidogrel bisulfate through an 80-mesh sieve for later use.
[0064] 2) Pass pregelatinized starch, low-substituted hydroxypropyl cellulose and micropowder silica gel through 80-mesh sieve respectively, and dry at 105°C for 2 hours for later use.
[0065] 3) Pass polyethylene glycol 6000 through a 60-mesh sieve for later use.
[0066] 4) Mix the prescription quantity I crystal form clopidogrel hydrogen sulfate, polyethylene glycol 6000, pregelatinized starch, low-substituted hydroxypropyl cellulose and 80% prescription quantity of micropowder silica gel evenly, pass through a dry granulator, an...
Embodiment 3
[0072] Raw material name 1000 tablets dosage (g)
[0073] Form I Clopidogrel Bisulfate 97.9 (equivalent to Clopidogrel 75g)
[0074] Polyethylene glycol 6000 2
[0075] Micronized silica gel 10
[0076] Microcrystalline Cellulose 150
[0077] Low-substituted hydroxypropyl cellulose 4
[0078]Gastric-soluble film coating premix appropriate amount
[0079] 1) Pass clopidogrel bisulfate through a 40-mesh sieve for later use.
[0080] 2) Pass the microcrystalline cellulose, low-substituted hydroxypropyl cellulose and micropowder silica gel through a 60-mesh sieve respectively, and dry at 105°C for 2 hours for later use.
[0081] 3) The polyethylene glycol 6000 is jet-pulverized for later use.
[0082] 4) Mix the prescription amount of clopidogrel hydrogen sulfate, microcrystalline cellulose, and low-substituted hydroxypropyl cellulose evenly, pass through a dry granulator, and granulate with a 20-mesh sieve.
[0083] 5) Pass the dry granules through a 18-mesh sieve for gran...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com