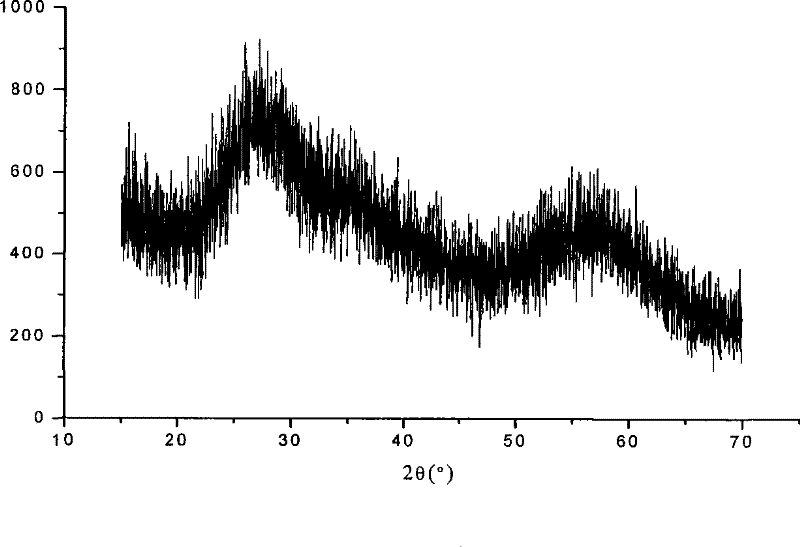
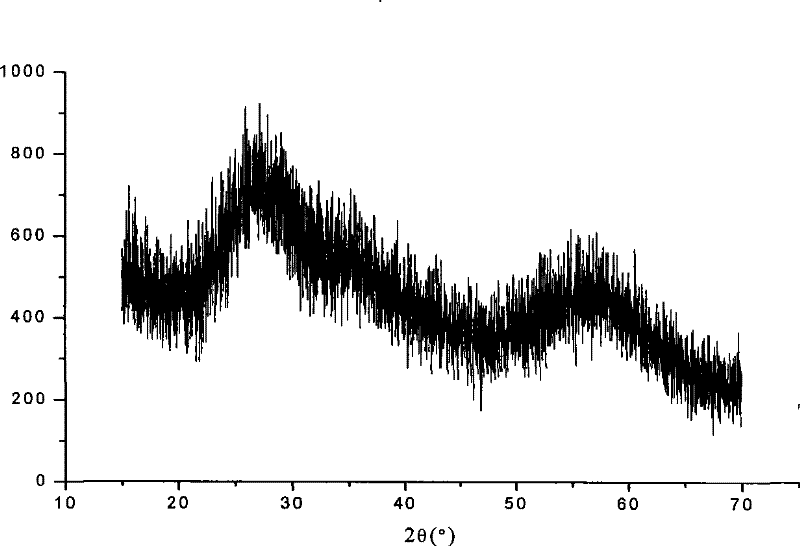
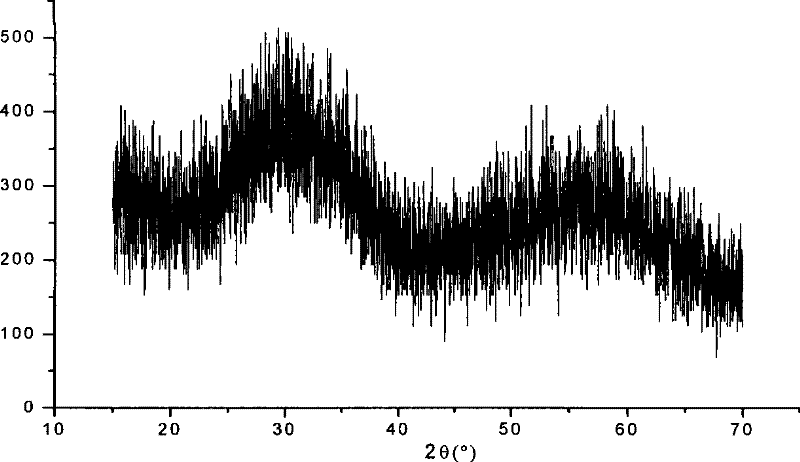
Preparation technology for hafnium carbonate
A technology of carbonic acid and hafnium sulfate, which is applied in the field of hydrometallurgy, can solve the problems that the preparation of hafnium carbonate has not been reported, and achieve the effect of high metal recovery and good solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Concentration [HfO 2 ]=292.15g / L, [H + ]=1.64mol / L hafnium oxychloride solution. Take 0.55L of this hafnium oxychloride solution, add 1.05L of water, and make the concentration [HfO 2 ]=100.4g / L feed liquid 1.6L. According to the molar ratio Hf 4+ : SO 4 2- = 1: 0.67 add H 2 SO 4 33 mL of analytically pure sulfuric acid with a content of 95% to 98%, add NH 3 60mL of analytically pure ammonia water with a content of 25% to 28%, the concentration of [HCl] neutralized into the solution is 1 to 1.5g / L, and stirred evenly. Divide into 4 beakers. Put it into a water bath for hydrolysis, and keep it warm at 95°C for 8 hours. After the reaction is finished, filter and wash the filter cake 4 to 5 times, and keep the filtered cake after washing.
[0023] Prepare 1.46L of 10% sodium carbonate solution. Put the filtered and dried filter cake into the sodium carbonate solution and stir evenly. Then put it into 4 beakers, put it into a water bath for transformation, and ...
Embodiment 2
[0033] Concentration [HfO 2 ]=349.83g / L, [H + ]=2.14mol / L hafnium oxychloride solution. Take this hafnium oxychloride solution 0.114L, add 0.286L water, make the concentration [HfO 2 ]=100.5g / L feed liquid 0.4L. According to the molar ratio Hf +4 : SO 4 2- = 1: 0.88 added H 2 SO 4 9.1 mL of analytically pure sulfuric acid with a content of 95% to 98%, add NH 3 15mL of analytically pure ammonia water with a content of 25% to 28%, the concentration of [HCl] neutralized into the solution is 1 to 1.5g / L, and stirred evenly. Put it into a water bath for hydrolysis, and keep it warm at 95°C for 8 hours. After the reaction is finished, filter and wash the filter cake 4 to 5 times, and keep the filtered cake after washing.
[0034] Prepare 0.36L of 10% sodium carbonate solution. Put the filter cake into the sodium carbonate solution and stir well. Put it into a water bath for transformation, and keep it warm at 85°C for 2 hours. After the reaction is finished, filter and ...
Embodiment 3
[0038] Concentration [HfO 2 ]=349.83g / L, [H + ]=2.14mol / L hafnium oxychloride solution. Take 0.46L of this hafnium oxychloride solution, add 1.14L of water, and make the concentration [HfO 2 ]=100.5g / L feed liquid 1.6L. According to the molar ratio Hf 4+ : SO 4 2- = 1: 0.88 added H 2 SO 4 36.4 mL of analytically pure sulfuric acid with a content of 95% to 98%, add NH 3 64mL of analytically pure ammonia water with a content of 25% to 28%, the concentration of [HCl] neutralized into the solution is 1 to 1.5g / L, and stirred evenly. Pour into 4 beakers. Put it into a water bath for hydrolysis, and keep it warm at 95°C for 8 hours. After the reaction is finished, filter and wash the filter cake 4 to 5 times, and keep the filtered cake after washing.
[0039] Prepare 1.16L of 10% ammonium bicarbonate solution. Put the filter cake into the ammonium bicarbonate solution and stir well. Put them into 4 beakers respectively, put them into a water bath for transformation, and...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com