Cisplatin spinal tumor slow-release implant and preparation method thereof
A technology for slow-release implants and spinal tumors, which is applied in the direction of anti-tumor drugs, pharmaceutical formulations, and medical preparations of non-active ingredients, etc. The effect of survival time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
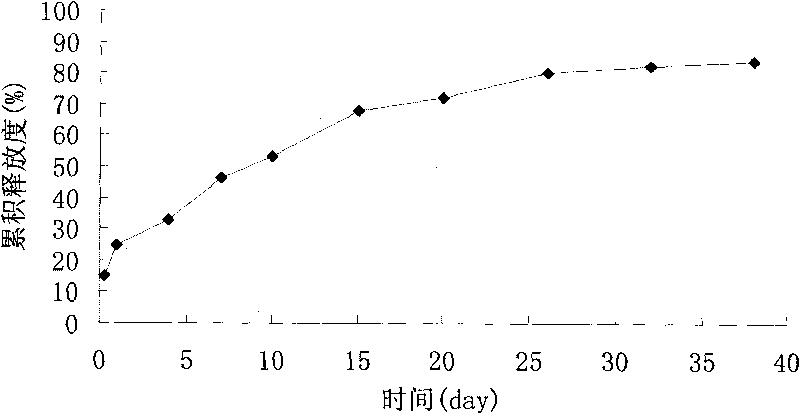
Embodiment 1
[0021] prescription:
[0022] Cisplatin: PLGA 30:70 (weight ratio)
[0023] PLGA concentration is 40% (50:50)
[0024] Oil phase solvent Dichloromethane
[0025] Emulsifier Polyvinyl alcohol
[0026] Accurately weigh 300 mg of cisplatin, dissolve it in an appropriate amount of double-distilled water, and use it as the inner water phase. Accurately weigh 700 mg of PLGA, dissolve in an appropriate amount of dichloromethane, and use it as the oil phase. The oil phase is added to the inner water phase under the condition of avoiding light and ultrasonication in an ice-water bath to obtain colostrum. Quickly add this colostrum into the polyvinyl alcohol solution; ultrasonically treat until the double emulsion is formed; then add the resulting double milk into the low-concentration polyvinyl alcohol solution. Remove dichloromethane by volatilization; collect the microspheres by centrifugation, wash with distilled water 3 times; add double distilled water to make a suspension, a...
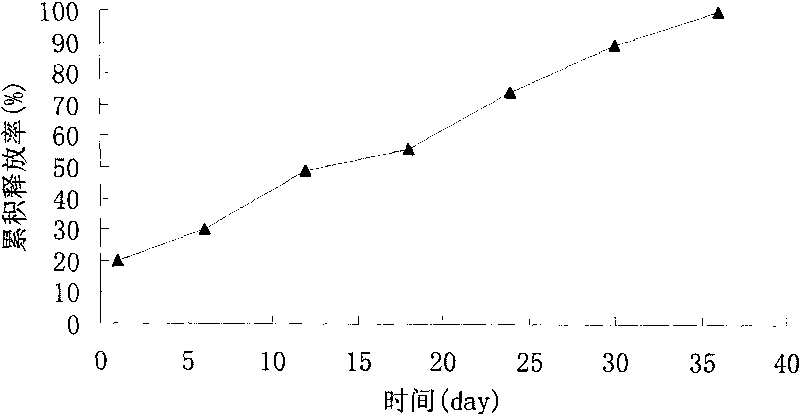
Embodiment 2
[0028] prescription:
[0029] Cisplatin: PLGA 25:75 (weight ratio)
[0030] PLGA concentration is 15% (75:25)
[0031] Oil phase solvent Dichloromethane
[0032] Emulsifier methyl cellulose
[0033] Accurately weigh 250 mg of cisplatin, dissolve it in an appropriate amount of double-distilled water, and use it as the inner water phase. Accurately weigh 750 mg of PLGA, dissolve in an appropriate amount of dichloromethane, and use it as the oil phase. The oil phase is added to the inner water phase under the condition of avoiding light and ultrasonication in an ice-water bath to obtain colostrum. This colostrum is quickly added to the methylcellulose solution; sonicated until the double emulsion is formed; then the resulting double emulsion is added to the low-concentration methylcellulose. The subsequent preparation process was the same as in Example 1 to prepare a cisplatin sustained-release implant.
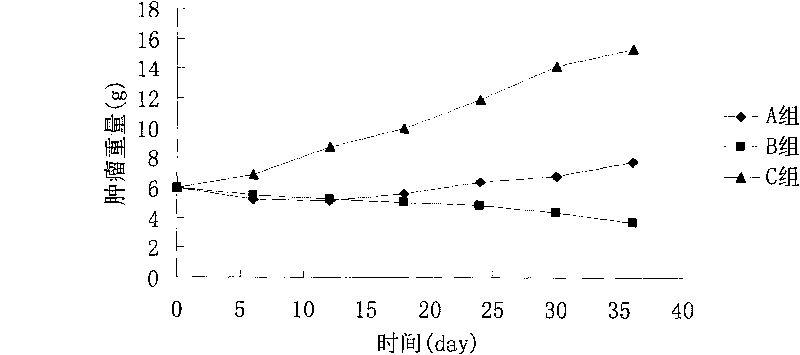
Embodiment 3
[0035] prescription:
[0036] Cisplatin: PLGA 20:80 (weight ratio)
[0037] PLGA concentration is 30% (50:50)
[0038] Oil phase solvent Dichloromethane
[0039] Emulsifier Polyvinyl alcohol
[0040] Accurately weigh 200 mg of cisplatin, dissolve it in an appropriate amount of double-distilled water, and use it as the inner water phase. Accurately weigh 800 mg of PLGA, dissolve in an appropriate amount of dichloromethane, and use it as the oil phase. The oil phase is added to the inner water phase under the condition of avoiding light and ultrasonication in an ice-water bath to obtain colostrum. The colostrum is quickly added to the polyvinyl alcohol solution; sonicated until the double emulsion is formed; then the resulting double emulsion is added to the low-concentration polyvinyl alcohol. The subsequent preparation process was the same as in Example 1 to prepare a cisplatin sustained-release implant.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com