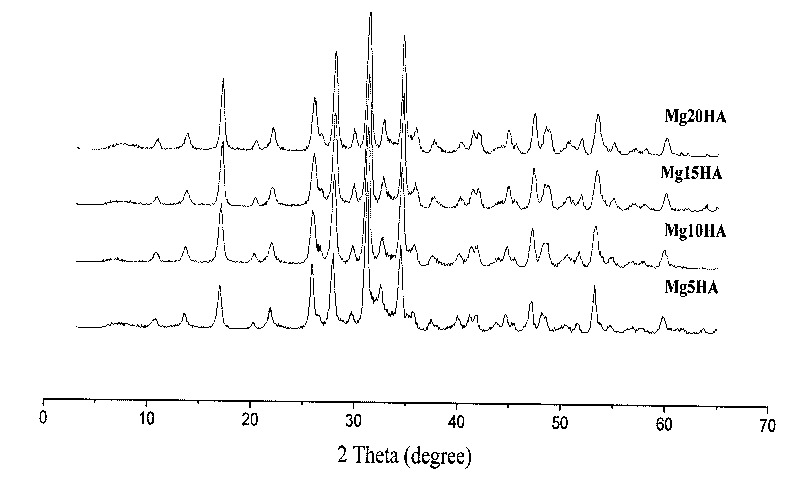
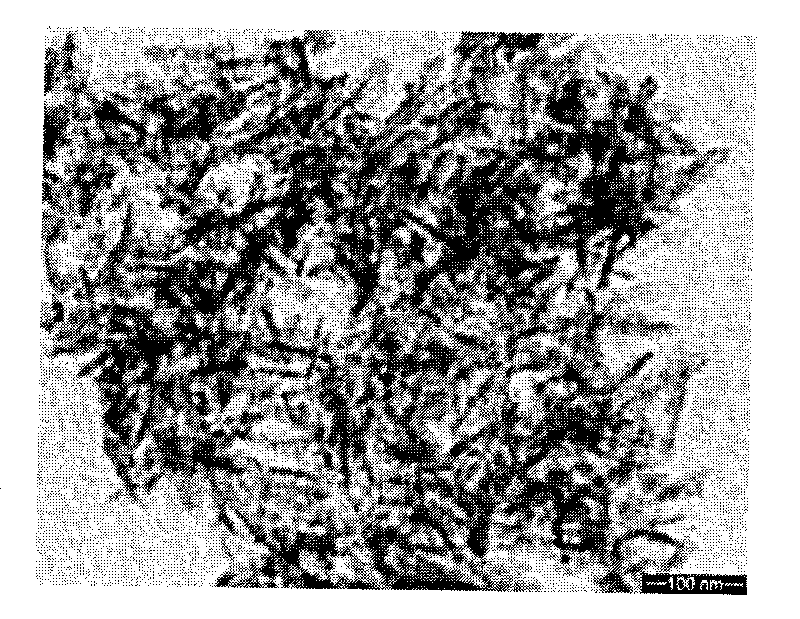Method for preparing nano magnesium whitlockite powder
A technology of magnesium white calcium phosphate and mineral powder, which is applied in the fields of chemical and biological materials, can solve the problems of rare crystal grain size and uniformity of magnesium white phosphate calcium ore, and achieves the effect of simple method and loose preparation conditions.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0027] The preparation method of the nano-magnesium calcium white phosphorous ore powder of the present invention, its preparation steps are:
[0028] (1) Ca(NO 3 ) 2 4H 2 O, Mg(NO 3 ) 2 ·6H 2 O, Na 2 EDTA·2H 2 O and Na 2 HPO 4 12H 2 O for material preparation;
[0029] (2) Na 2 EDTA·2H 2 O, Ca(NO 3 ) 2 4H 2 O and Mg(NO 3 ) 2 ·6H 2 Dissolve O together in water to obtain 1# solution, adjust the pH value and concentration to finally make the pH value of 1# solution 7, and the concentration is 4~5mol.L -1 ;
[0030] (3) Na 2 HPO 4 12H 2 O is dissolved in water to form a solution, and the pH value is adjusted to be consistent with the 1# solution to obtain the 2# solution, and the volume of the 2# solution is the same as the 1# solution volume;
[0031] (4) Slowly add the 2# solution to the 1# solution, and stir while adding. The purpose of slowly adding and stirring is to fully react the 2# solution and the 1# solution to obtain the 3# solution;
[0032] (...
Embodiment 1
[0043] Weigh 18.6 g Na 2 EDTA·2H 2 O powder, 112.2 grams of Ca(NO 3 ) 2 4H 2 O and 6.4 g Mg(NO 3 ) 2 ·6H 2 O powder, dissolve into 40 milliliters of deionized water successively and fully stir to dissolve, use 50 milliliter volumetric flasks to constant volume, prepare and obtain 1# solution; Weigh 107.4 grams of Na 2 HPO 4 12H 2 O was dissolved in 40 milliliters of deionized water and stirred until completely dissolved to prepare 2# solution; the 1# and 2# solutions were adjusted to pH=7 with the 5M NaOH solution prepared in advance, and 2# was mixed under the condition of vigorous stirring. # solution was added dropwise to 1# solution, and 100 ml of mixed solution was obtained after the addition was completed; adjust the pH value of the solution to 7, then magnetically stirred the 100 ml mixed solution at a constant temperature of 60°C for 2 hours, and then put the mixed solution in polytetrafluoroethylene In the ethylene reaction kettle, make it carry out the hydro...
Embodiment 2
[0045] Weigh 18.6 g Na 2 EDTA·2H 2 O powder, 106.2 grams of Ca(NO3) 2 4H 2 O and 12.8 g Mg(NO 3 ) 2 ·6H 2 O powder, dissolve into 40 milliliters of deionized water successively and fully stir to dissolve, use 50 milliliter volumetric flasks to constant volume, prepare and obtain 1# solution; Weigh 107.4 grams of Na 2 HPO 4 12H 2 O was dissolved in 40 milliliters of deionized water and stirred until completely dissolved to prepare 2# solution; the 1# and 2# solutions were adjusted to pH=7 with the 5M NaOH solution prepared in advance, and 2# was mixed under the condition of vigorous stirring. # solution was added dropwise to 1# solution, and after the dropwise addition was completed, 100 ml of mixed solution was obtained, and the pH of the mixed solution was adjusted to 7 with 5M NaOH solution; then 100 ml of mixed solution was magnetically stirred at a constant temperature of 60°C for 2 hours, and then mixed Put the liquid into a polytetrafluoroethylene reactor, and ma...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com