Method for preparing D-serine by kinetic resolution
A kinetic resolution, serine technology, applied in chemical instruments and methods, cyanide reaction preparation, preparation of organic compounds, etc., to achieve the effect of improving the separation yield, reducing operating costs, and stable product quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
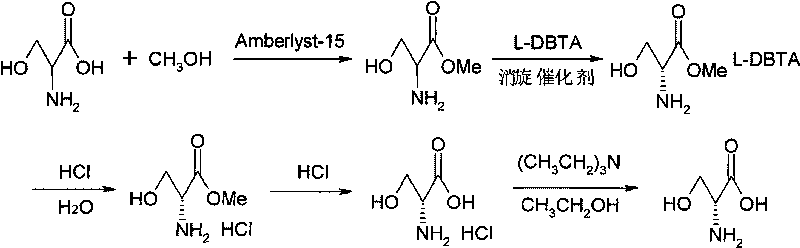
[0027] (1) Preparation of DL-serine methyl ester:
[0028] Add 84.0g (0.80mol) DL-serine, 400ml anhydrous methanol and 252.0g Amberlyst-15 ion exchange resin to the reactor with stirring, heating and thermometer, heat up to 65°C while stirring, and maintain the temperature React for 1 hour. The reaction solution was cooled to room temperature, filtered, and reused after the filtrate was recovered, and the solid phase was adjusted to pH=8 with 30% ammonia water, extracted 3 times with each 200ml ethyl acetate solvent, and the ethyl acetate solvent was evaporated (recycled after solvent recovery) ) to obtain 91.6 g of DL-serine methyl ester, with a yield of 96.2% and a purity of 99.36% (HPLC).
[0029] (2) Preparation of D-serine methyl ester L-DBTA di-salt:
[0030] Add respectively the DL-serine methyl ester that 23.8g (0.20mol) step (1) obtains in the reactor with stirring, heating, thermometer, 50ml methanol, 1.1g (0.01mol) 2-formylpyridine, while stirring Heat up to 60°C...
Embodiment 2
[0034] (1) Preparation of DL-serine methyl ester is the same as in Example 1.
[0035] (2) Preparation of D-serine methyl ester L-DBTA di-salt:
[0036] Add 23.8g (0.20mol) of DL-serine methyl ester obtained in step (1) respectively, 180ml methanol, 2.1g (0.02mol) 4-formylpyridine in a reactor with stirring, heating, and a thermometer, while stirring Heat up to 35°C, add dropwise L-DBTA methanol solution [prepared from 35.8g (0.10mol) L-DBTA and 72ml methanol] within 3 hours, react at the same temperature for 3 hours after dropping, and cool the reaction solution to room temperature , filtered, and the filtrate was used mechanically. The crystals were rinsed with 30ml of methanol solvent for 3 times and then dried to obtain 47.1g of crystalline D-serine methyl ester L-DBTA disalt. The resolution yield was 79.2%, and the melting point was 168.5°C. Specific rotation
[0037] (3) Preparation of D-serine:
[0038] With the method of step (3) of Example 1, take 45.0g (0.075m...
Embodiment 3
[0040] (1) Preparation of DL-serine methyl ester is the same as in Example 1.
[0041] (2) Preparation of D-serine methyl ester L-DBTA di-salt:
[0042]Add respectively 23.8g (0.20mol) of DL-serine methyl ester obtained in step (1), 180ml methanol, 0.37g (0.002mol) 2-bromo-4-formylpyridine in a reactor with stirring, heating and a thermometer , heated up to 55°C while stirring, added dropwise L-DBTA methanol solution [prepared from 35.8g (0.10mol) L-DBTA and 140ml methanol] within 1 hour, and reacted at the same temperature for 1.5 hours after the drop was completed. The liquid was cooled to room temperature, filtered, and the filtrate was used mechanically. The crystals were washed with 30ml methanol solvent for 3 times and then dried to obtain 52.2g of crystalline D-serine methyl ester·L-DBTA di-salt. The resolution yield was 87.8%. : 168.1℃, specific rotation
[0043] (3) Preparation of D-serine:
[0044] With the method of step (3) of Example 1, take 50.0g (0.084mol) ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com