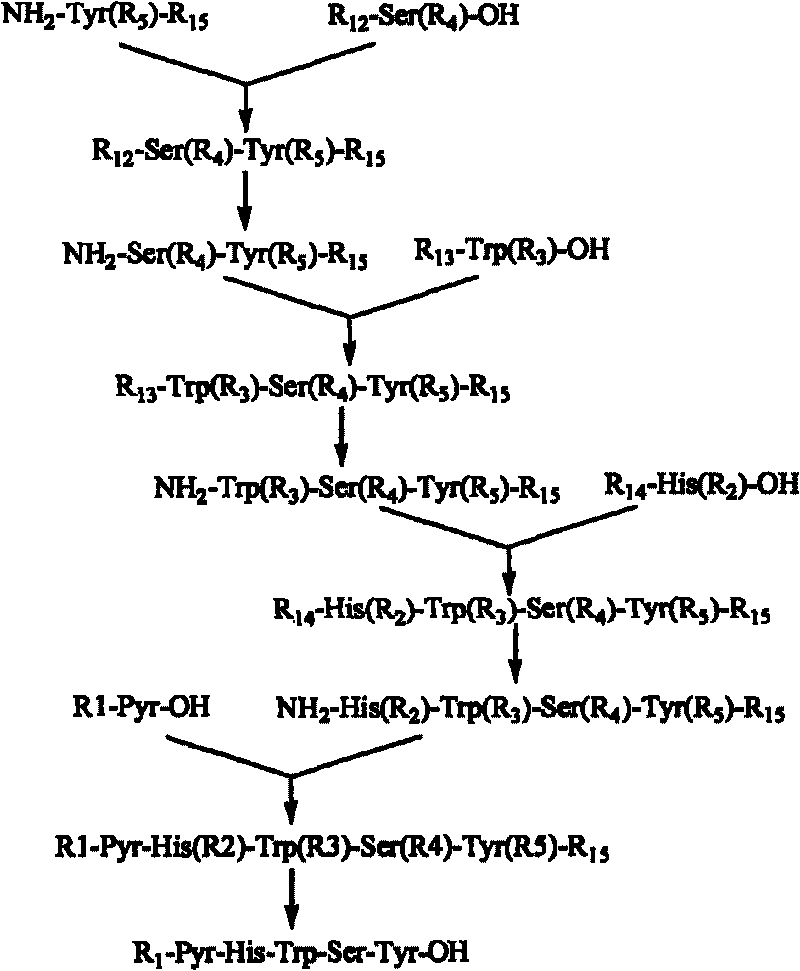Synthesizing method of buserelin
A synthesis method and a condensing agent technology, which are applied in the field of pure liquid-phase fragment synthesis of buserelin, can solve problems such as being unsuitable for industrial production, and achieve the effects of shortening the synthesis cycle, mild reaction conditions, and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
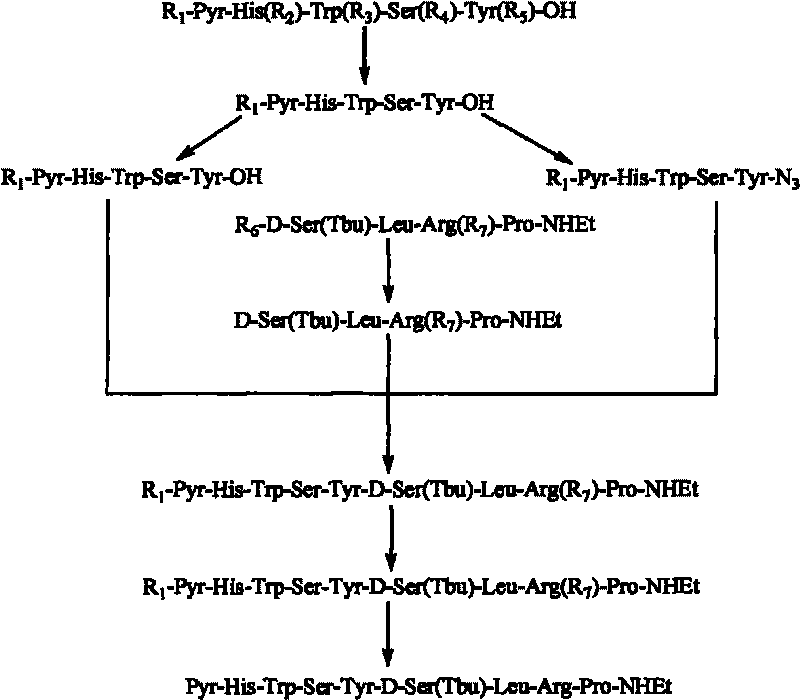
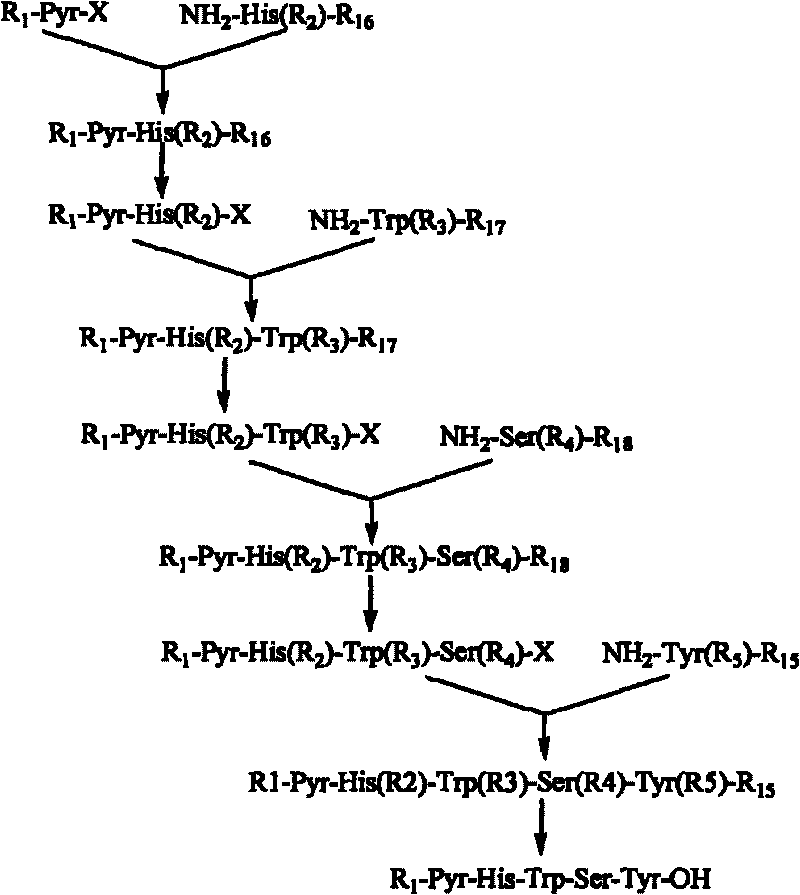
[0063] Embodiment 1: Synthetic pentapeptide fragment Pyr-His-Trp-Ser-Tyr-OH
[0064] 1. Synthesis of Fmoc-Ser(Tbu)-Tyr-OMe:
[0065] Add Fmoc-Ser(Tbu)-OH (20mmol), NH2-Tyr-OMe (20mmol), HOSU (22mmol) in the round bottom flask of 50 milliliters, dissolve with 40 milliliters of anhydrous DMF, add DCC (22mmol) under ice-water bath ), stirred at room temperature for 2 hours, and the detection reaction was complete. The precipitate produced by the reaction was removed by suction filtration, concentrated under reduced pressure to remove DMF, and then dissolved with a large amount of ethyl acetate, and then dissolved with NaHCO 3 Washing, washing with dilute hydrochloric acid, washing with saturated brine, drying over anhydrous sodium sulfate, and spin-drying ethyl acetate gave a pale yellow solid (Fmoc-Ser(Tbu)-Tyr-OMe).
[0066] HPLC purity: more than 92, the yield is 90.5MS=561 (M+).
[0067] 2. Synthesis of Fmoc-Trp(Boc)-Ser(Tbu)-Tyr-OMe:
[0068] Weigh Fmoc-Ser(Tbu)-Tyr-OMe ...
Embodiment 2
[0083] Example 2 Synthesis of Tetrapeptide Fragment D-Ser(Tbu)-Leu-Arg-Pro-NHEt
[0084] 1. Synthesis of Fmoc-Pro-NHEt
[0085] Weigh Fmoc-Pro-OH (20mmol) and place it in a 100ml round bottom flask, dissolve it with 55ml DMF, then add HOSU (22mmol), add 20ml DMF solution of DCC (22mmol) under ice-water bath, and react at room temperature For 4 hours, filter off the precipitate, concentrate the solution to 40 ml, add 20 ml of concentrated ethylamine aqueous solution, and react overnight at room temperature. Washing, washing with brine, drying over anhydrous sodium sulfate, and spin-drying the solvent to obtain a light yellow solid (Fmoc-Pro-NHEt).
[0086] HPLC purity: greater than 92, yield 81% MS = 365 (M+).
[0087] 2. Synthesis of Boc-Arg(NO2)-Pro-NHEt
[0088] Weigh Fmoc-Pro-NHEt (20mmol) and place it in a 100ml round-bottomed flask, add 30ml of piperidine / DMF 25% solution, react at room temperature for 30 minutes, detect that the reaction is complete, add a large amoun...
Embodiment 3
[0102] The synthesis of embodiment 3Pyr-His-Trp-Ser-Tyr-Ser(Tbu)-Leu-Arg-Pro-NHEt
[0103] Add Pyr-His-Trp-Ser-Tyr-OH (20mmol) in a 50ml round bottom flask, dissolve with 40ml of anhydrous DMF, add tert-butyl chloroformate (8ml) under ice-salt bath, react 5-20 Minutes, 20 ml of DMF solution of NH2-Ser(Tbu)-Leu-Arg-Pro-NHEt (20mmol) was added, reacted in ice-salt bath for 60 minutes, stirred at room temperature for 12 hours, and detected that the reaction was complete. The precipitate produced by the reaction was removed by suction filtration, and concentrated under reduced pressure to remove DMF to obtain a light yellow crude product (Pyr-His-Trp-Ser-Tyr-Ser(Tbu)-Leu-Arg-Pro-NHEt).
[0104] HPLC purity: greater than 85, yield 72% MS=1240 (M+1). The HPLC spectrum of the crude product of Buserelin is as follows Image 6 shown.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Wavelength | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com