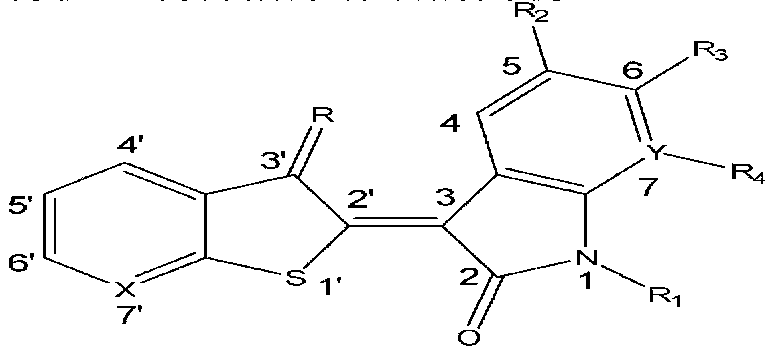
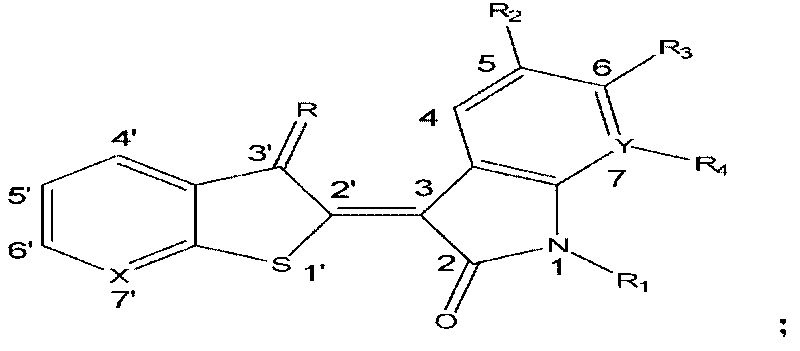
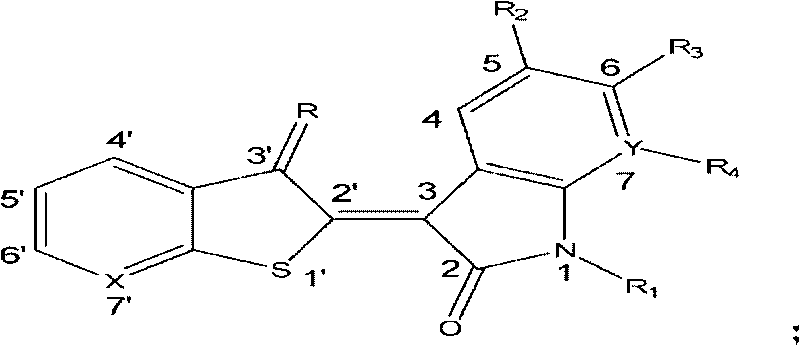
1'-thio-aza indirubin compound, application and preparation method thereof
A technology of indirubin and compounds, which is applied in the field of medicine, can solve the problems that the anti-tumor effect of indirubin compounds has not been investigated, and achieve the effect of inhibiting or killing tumor cells
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] 1′-Thio-7′-azaidirubin
[0049] Add 20mL methanol, isatin (76mg, 0.52mmol), thieno[2,3-b]pyridin-3-one (78mg, 0.52mmol) and anhydrous sodium carbonate (137mg, 1.29mmol) successively in a 50mL single-necked flask , stirred at room temperature for 6 h under nitrogen protection, and brown flocculent precipitates gradually precipitated. After filtration, the filter cake was washed successively with 50 mL of methanol, 50 mL of distilled water and 50 mL of methanol, and dried to obtain 125 mg of brown flocculent solid, yield 86.4%, mp>300°C. 1 H-NMR (300MHz, DMSO, δppm): 11.20 (s, 1H, NH), 8.99 (d, 1H, J=8.1Hz, H-4), 8.79 (dd, 1H, J=1.5Hz, 4.5Hz, H-6'), 8.22(dd, 1H, J=1.5Hz, 7.8Hz, H-4'), 7.40-7.49(m, 2H, H-6, 5'), 7.10(t, 1H, J= 8.1 Hz, H-5), 6.96 (d, 1H, J=7.8 Hz, H-7). IR (cm -1 , KBr): 3128, 3059, 1709, 1674, 1612, 1573, 1462, 1399, 1332, 1293, 1286, 1223, 1059, 1025, 1003, 761, 750, 740. ESI-MSm / z: 281.16[M+H] + .
Embodiment 2
[0051] 6-Fluoro-1′-thio-7′-azaidirubin
[0052] By 6-fluoroisatin and thieno [2,3-b] pyridin-3-one reaction in the system. Method is with embodiment 1.
[0053] Reddish-brown flocculent solid, yield 70.5%, mp>300°C. 1 H-NMR (300MHz, DMSO, δppm): 11.38(s, 1H, NH), 9.02-9.07(m, 1H, H-4), 8.79(dd, 1H, J=1.8Hz, 4.5Hz, H-6 '), 8.21(dd, 1H, J=1.8Hz, 7.5Hz, H-4'), 7.44-7.49(m, 1H, H-5'), 6.92(t, 1H, J=9.0Hz, H- 5), 6.77 (dd, 1H, J=2.7Hz, 9.0Hz, H-7). IR (cm -1 , KBr): 3119, 3073, 1722, 1678, 1621, 1578, 1498, 1450, 1408, 1333, 1298, 1153, 1136, 1055, 1005, 967, 846, 760, 743. ESI-MS m / z: 299.15[M+H] + .
Embodiment 3
[0055] 6-Chloro-1′-thio-7′-azaidirubin
[0056] By 6-chloro isatin and thieno [2,3-b] pyridin-3-one reaction in the system. Method is with embodiment 1.
[0057] Reddish-brown flocculent solid, yield 71.3%, mp>300°C. 1 H-NMR (300MHz, DMSO, δppm): 11.38(s, 1H, NH), 8.99(d, 1H, J=8.1Hz, H-4), 8.80(d, 1H, J=5.1Hz, H-6 '), 8.23 (d, 1H, J=8.4Hz, H-4'), 7.45-7.50 (m, 1H, H-5'), 7.18 (d, 1H, J=8.1Hz, H-5), 6.99 (s, 1H, H-7). IR (cm -1 , KBr): 3119, 3074, 1722, 1679, 1613, 1576, 1454, 1443, 1406, 1326, 1294, 1098, 1050, 1003, 929, 853, 817, 758, 742. ESI-MS m / z: 313.2, 315.1 [M+H] + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com