Organic molecular line and preparation method
A technology of organic molecules and molecular wires, which is applied in the field of preparing ordered tetrathiafulvalene or derivative molecular wires on the surface of high-temperature pyrolytic graphite, achieving the effects of simple process, low cost and good electrical properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] 1) In a 1.5mL centrifuge tube, add about 0.5mg of TTF to 1mL of n-tetradecane solvent (concentration is about 0.5mg / mL), and use an ultrasonic instrument (power of 100W) to sonicate for more than 10 minutes to ensure that TTFs are uniform Dispersed and dissolved in a solvent;
[0043] 2) Treat the HOPG substrate: select a graphite sheet with a flat surface, and stick off a layer of the surface with an adhesive tape to obtain an atomically flat HOPG surface;
[0044] 3) After transferring to the dropper, drop a drop of solution (about 1 μL) onto the clean surface of HOPG. Since both graphite and the solution are hydrophobic, the solution diffuses on the graphite surface to form a film with uniform thickness, and TTFs molecules adsorb on the surface of HOPG to form molecular lines.
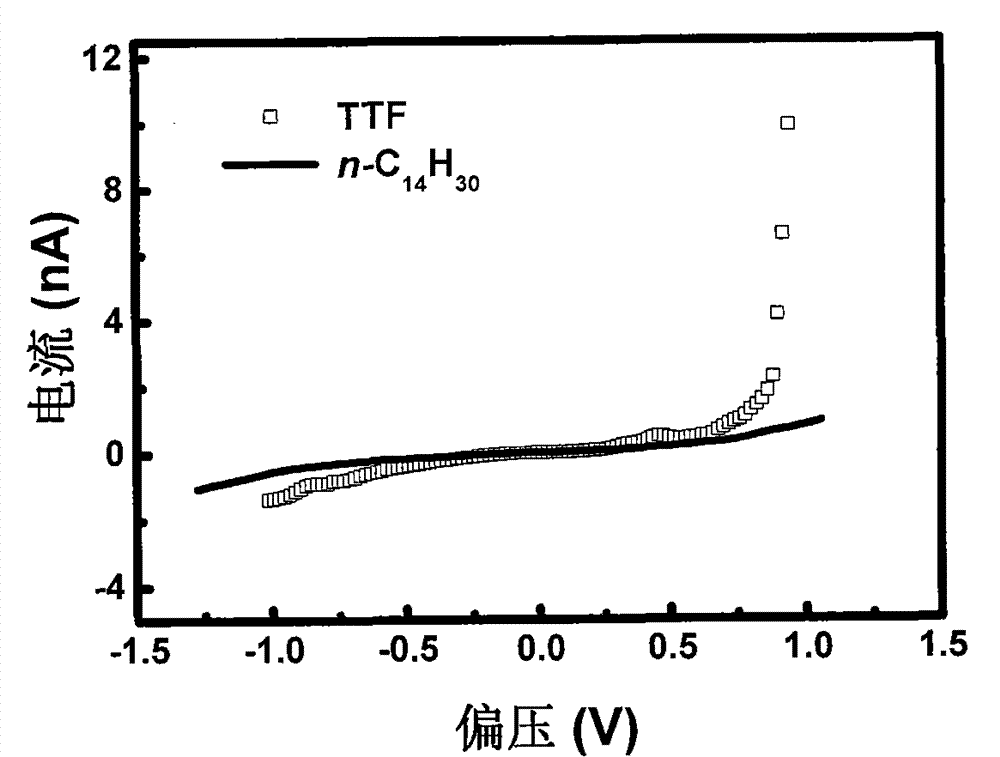
[0045] In order to use a scanning tunneling microscope (STM) to measure the molecular wires prepared in this example, the HOPG dripped with the above solution was first fixed on the sample st...
Embodiment 2
[0049] In a 1.5mL centrifuge tube, add about 0.5mg of DP-TTF to 1mL of n-tetradecane (concentration is about 0.5mg / mL), and ultrasonically disperse for about ten minutes to completely dissolve the molecule. After transferring to the dropper, drop a drop of the solution (approximately 1 μL) onto the clean HOPG surface (newly cleaved HOPG). Since graphite and the solution are both hydrophobic, the solution diffuses on the graphite surface to form a film of uniform thickness. In order to measure with a scanning tunneling microscope (STM), the HOPG dripped with the above solution was first fixed on the sample stage, and then the STM needle tip (Pt / Ir, 80:20) was manipulated to slowly approach the sample surface until it was immersed in the film. Do not touch the graphite surface, then scan to obtain the STM image of the DP-TTF-n-tetradecane molecule adsorbed at the solid-liquid interface (reference Figure 2A , Figure 2B ). In order to reduce or avoid the influence of the needl...
Embodiment 3
[0053] In a 1.5mL centrifuge tube, add about 0.05mg of DN-TTF to 1mL of n-tetradecane (concentration is about 0.05mg / mL), and ultrasonically disperse for about ten minutes to completely dissolve the molecule. After transferring to the dropper, drop a drop of the solution (approximately 1 μL) onto the clean HOPG surface (newly cleaved HOPG). Since graphite and the solution are both hydrophobic, the solution diffuses on the graphite surface to form a film of uniform thickness. In order to measure with a scanning tunneling microscope (STM), the HOPG dripped with the above solution was first fixed on the sample stage, and then the STM needle tip (Pt / Ir, 80:20) was manipulated to slowly approach the sample surface until it was immersed in the film. Do not touch the graphite surface, then scan to obtain the STM image of the DN-TTF-n-tetradecane molecule adsorbed at the solid-liquid interface (reference Figure 3A , Figure 3B ). In order to reduce or avoid the influence of the nee...
PUM
| Property | Measurement | Unit |
|---|---|---|
| length | aaaaa | aaaaa |
| width | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com