Leaching method of vanadium in vanadium-containing stone coal
A vanadium stone and leaching technology, which is applied in the field of vanadium extraction, can solve the problems of environmental pollution, vanadium leaching rate decline, loss, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
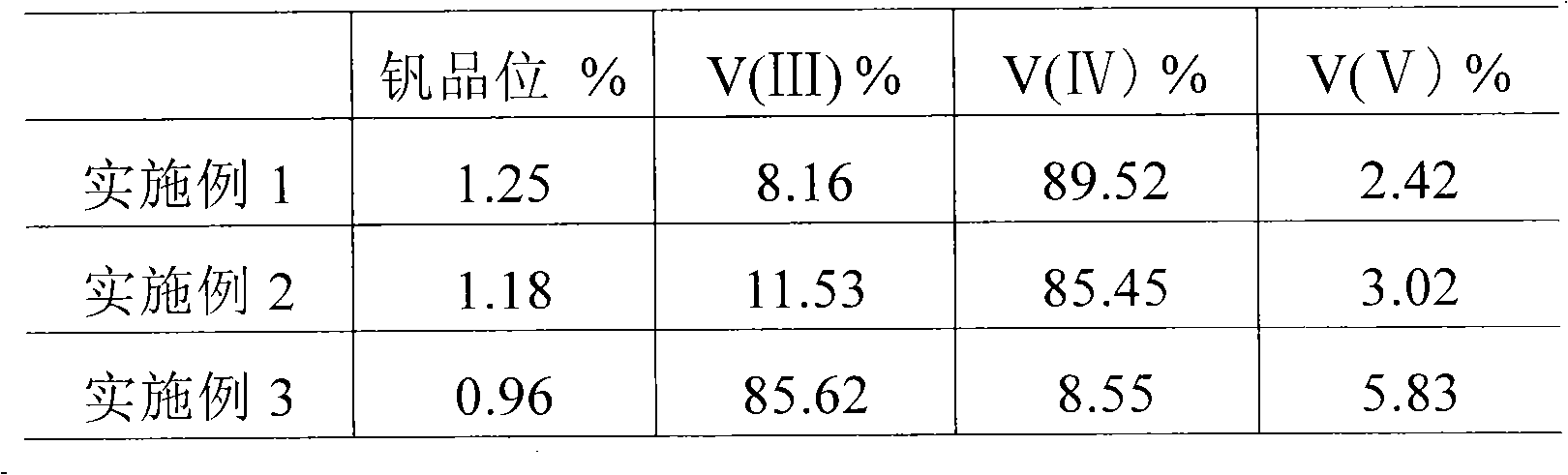
[0018] ① 100g vanadium-containing stone coal containing 1.25% vanadium is crushed, ball-milled to 80 mesh, and then added to the reaction kettle, 12g 98% sulfuric acid and 2.5g fluorite are added, and water is added to control the liquid-solid weight ratio to 1:1, and react at 90°C for 10 hours Then, liquid-solid separation was performed to obtain 100 g of leachate.
[0019] ②After adjusting the pH of the leaching solution to 2.5 with lime milk, at room temperature, extract the leaching solution with 100g 10% P204 and 5% TBP extractant diluted with kerosene at 7 levels, and the raffinate is returned to the reactor with 7 level sulfuric acid with a concentration of 3N The organic phase is loaded by back extraction to obtain a back extraction solution rich in vanadium ions.
[0020] ③After adding 0.75g sodium chlorate to the stripping solution, adding 12% ammonia water to adjust the pH to 2.2, filtering and washing the precipitate, calcining the precipitate in an electric calciner at...
Embodiment 2
[0023] ① 100g vanadium-containing stone coal containing 1.18% vanadium is crushed, ball-milled to 80 mesh, and then added to the reactor, 20g 98% sulfuric acid, 2.5g fluorite and 0.2g sodium chlorate are added, and water is added to control the liquid-solid weight ratio to 1:1 After reacting at 90°C for 2h, add 1g of fluorite, and after leaching at 90°C for 10h, liquid-solid separation to obtain 100g of leaching solution.
[0024] ②After adjusting the pH of the leaching solution to 2.5 with lime milk, at room temperature, the leaching solution is extracted with 100g 10% P204 and 5% TBP extractant diluted with kerosene at 7 levels, and the raffinate is returned to the reactor with 2N sulfuric acid 7 The organic phase is loaded by stage back extraction to obtain a back extraction liquid rich in vanadium ions.
[0025] ③After adding 0.65g sodium chlorate to the stripping solution, adding 12% ammonia water to adjust the pH to 2.2, filtering and washing the precipitate, calcining the pr...
Embodiment 3
[0028] ① 100g vanadium-containing stone coal containing 0.96% vanadium is crushed, ball-milled to 80 mesh, and then added to the reaction kettle, 25g 98% sulfuric acid, 4.0g fluorite and 0.3g sodium chlorate are added, and water is added to control the liquid-solid weight ratio to 1:1 After reacting at 90°C for 2h, add 2g of fluorite. After reacting for 2h, add the remaining 2g of fluorite. After leaching at 90°C for 10h, liquid-solid separation yields 100g of leachate.
[0029] ②After adjusting the pH of the extract with lime milk to 2.5, at room temperature, extract the extract with 100g of 10% P204 and 5% TBP extractant diluted with kerosene at 7 levels. The raffinate is returned to the reaction kettle with 7 levels of 2N sulfuric acid. The organic phase is loaded by back extraction to obtain a back extraction solution rich in vanadium ions.
[0030] ③After adding 0.60g of sodium chlorate to the stripping solution, adding 12% ammonia water to adjust the pH to 2.2, filtering and ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com