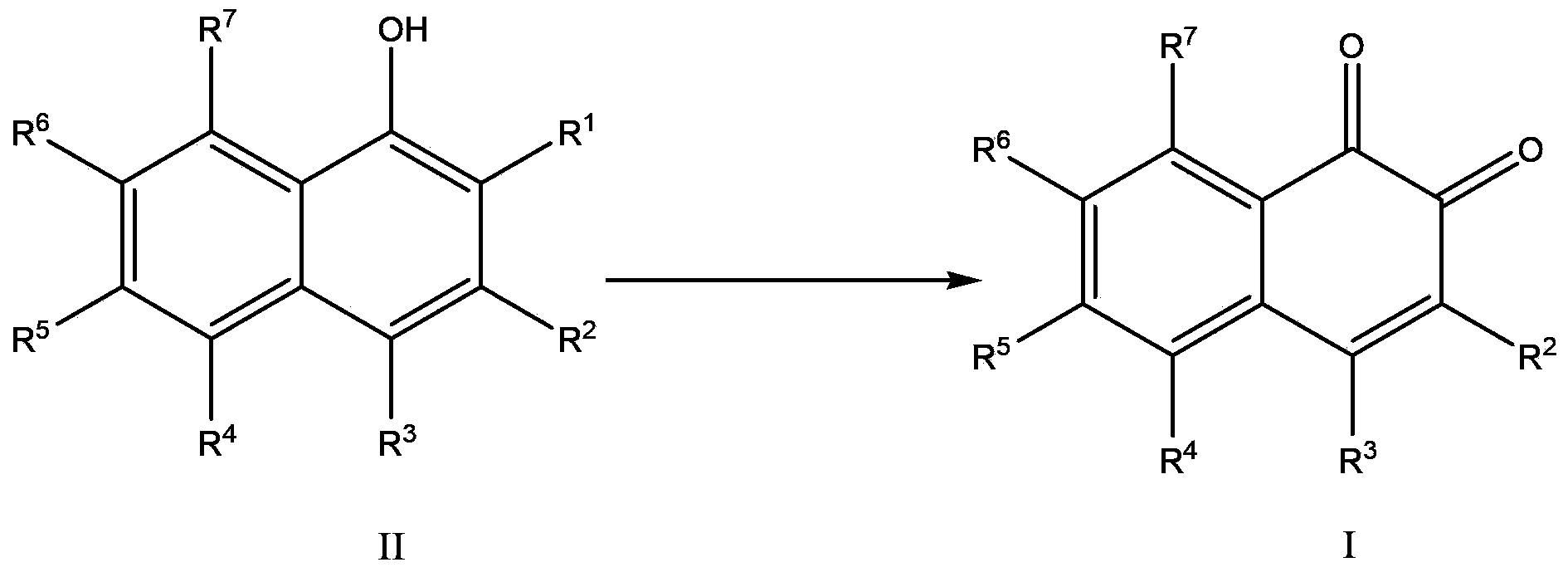
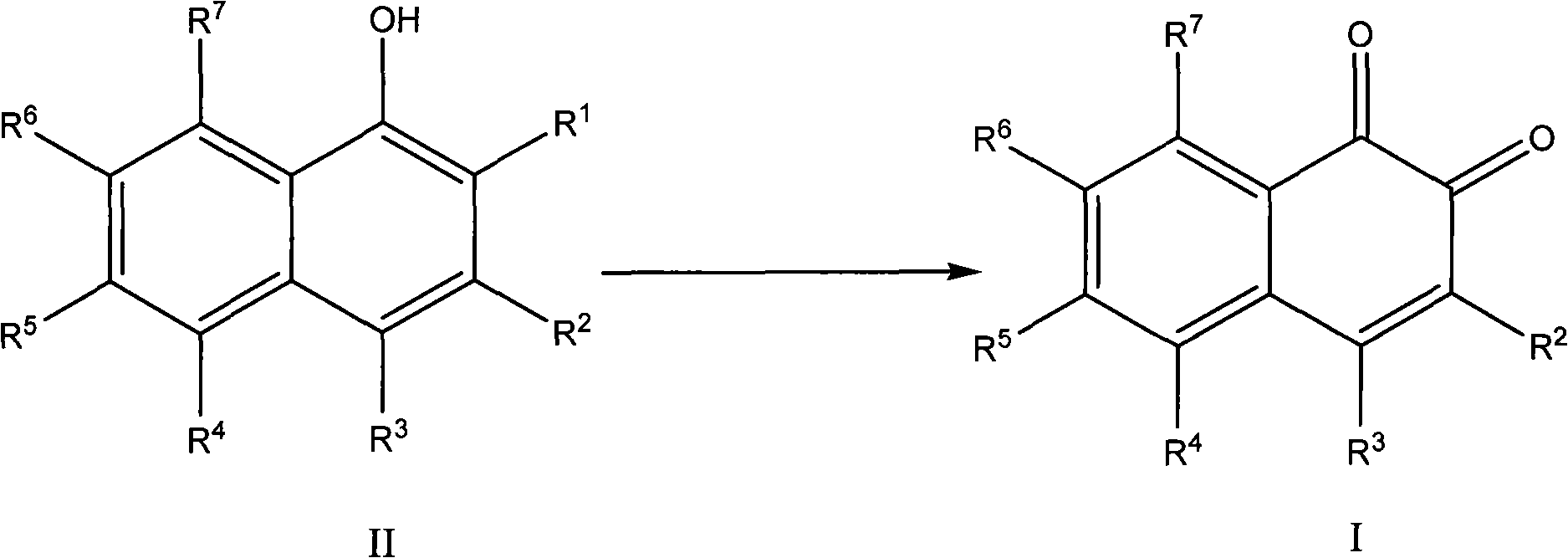
Method for preparing 1,2-naphthoquinone compound
A compound, naphthoquinone technology, applied in the field of preparation of chemical intermediates, can solve problems such as harsh labor protection conditions, difficulty in large-scale industrial production, and complex processing, and achieve the effects of high yield, low cost, and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 14
[0031] The preparation of embodiment 14-chloro-1,2-naphthoquinone
[0032] Add 2,4-dichloro-α-naphthol (21.3g, 0.1mol), acetic acid (400mL), sodium periodate (21.4g, 0.1mol) to the reaction flask in sequence at room temperature (25-30°C), Then stir and heat to 40°C and react until the 2,4-dichloro-α-naphthol disappears as traced by TLC. After the reaction, the reaction solution was cooled to room temperature, and water (300 mL) was added to dissolve unreacted sodium periodate. It was extracted with ethyl acetate (400 mL), dried and concentrated to give an orange solid with a yield of 82% and a purity of 99.4% (HPLC).
Embodiment 24
[0033] The preparation of embodiment 24-chloro-1,2-naphthoquinone
[0034] Add 2,4-dichloro-α-naphthol (21.3g, 0.1mol), propionic acid (400mL), sodium periodate (42.8g, 0.2mol) to the reaction flask in sequence at room temperature (25-30°C) , followed by stirring and heating to 50 ° C. The reaction was followed directly by TLC to complete the reaction of 2,4-dichloro-α-naphthol. After the reaction, the reaction liquid was cooled to room temperature, and water (300 mL) was added to dissolve unreacted sodium periodate. It was extracted with ethyl acetate (400 mL), dried and concentrated to give an orange solid with a yield of 85% and a purity of 99.2% (HPLC).
Embodiment 3
[0035] Example 3 The preparation of 4-chloro-1,2-naphthoquinone
[0036] Add 2,4-dichloro-α-naphthol (21.3g, 0.1mol), butyric acid (500mL), sodium periodate (64.2g, 0.3mol) to the reaction flask in sequence at room temperature (25-30°C) , followed by stirring and heating to 55 ° C. The reaction was followed directly by TLC to complete the reaction of 2,4-dichloro-α-naphthol. After the reaction, the reaction liquid was cooled to room temperature, and water (300 mL) was added to dissolve unreacted sodium periodate. It was extracted with ethyl acetate (400 mL), dried and concentrated to give an orange solid with a yield of 80% and a purity of 99.6% (HPLC).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com