Method for preparing 4-methyl-2-propyl-1H-benzimidazole-6-formamide compound
A technology of amine compounds and benzimidazole, which is applied in the field of chemical synthesis, can solve the problems of low product purity, large environmental pollution, and low reaction yield, and achieve the effects of good purity, low environmental pollution, and high product yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
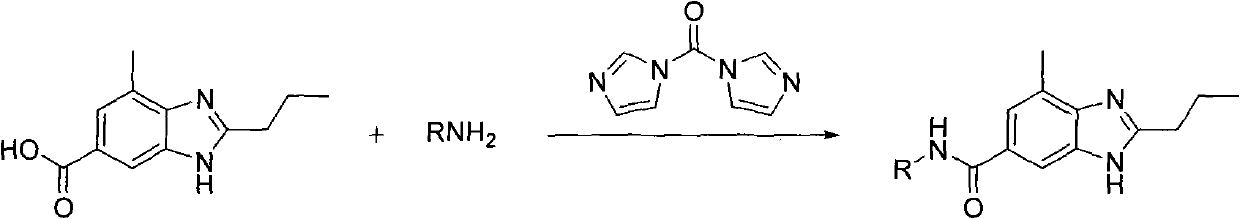
[0026] Example 1 Preparation of 4-methyl-2-propyl-N-propyl-1H-benzimidazole-6-carboxamide
[0027] Add 5.0g (23mmol) of 4-methyl-2-propyl-1H-benzimidazole-6-carboxylic acid and 20mL of N,N-dimethylformamide into a 500mL three-necked flask, and add N,N'- Carbonyldiimidazole 3.7g (23mmol), stirred at room temperature for 30min, then added n-propylamine 1.3g (23mmol), continued to stir at 100°C for 4h after the addition, evaporated the solvent under reduced pressure, added water to the residue and stirred until the solid precipitated completely. Suction filtration and drying yielded 5.9 g, yield 94%. 1 H NMR (DMSO-d 6 , 400MHz) δ: 0.91(t, J=7.25Hz, 3H), 0.95(t, J=7.24Hz, 3H), 1.59(m, 2H), 1.76(m, 2H), 2.55(s, 3H), 2.78(t, J=7.24Hz, 2H), 3.21(m, 2H), 7.47(s, 1H), 7.81(s, 1H).
Embodiment 2
[0028] Example 2 Preparation of 4-methyl-2-propyl-N-propyl-1H-benzimidazole-6-carboxamide
[0029] Add 5.0 g (23 mmol) of 4-methyl-2-propyl-1H-benzimidazole-6-carboxylic acid into a 500 mL three-necked flask, 30 mL of dichloromethane, and add N, N'-carbonyldiimidazole 3.7 g ( 23mmol), stirred at room temperature for 30min, then added 1.3g (23mmol) of propylamine, continued to stir at room temperature for 12h after the addition, and evaporated the solvent. Add water to the residue and stir until the solid precipitates out completely. Suction filtration and drying yielded 5.5 g with a yield of 93%. 1 H NMR (DMSO-d 6 , 400MHz) δ: 0.91(t, J=7.25Hz, 3H), 0.95(t, J=7.24Hz, 3H), 1.59(m, 2H), 1.76(m, 2H), 2.55(s, 3H), 2.78(t, J=7.24Hz, 2H), 3.21(m, 2H), 7.47(s, 1H), 7.81(s, 1H).
Embodiment 34
[0030] Preparation of Example 34-methyl-2-propyl-N-isopropyl-1H-benzimidazole-6-carboxamide
[0031] The amine compound uses 23mmol isopropylamine, and other feeding and implementation methods are the same as in Example 2. 5.4 g of white solid was obtained with a yield of 91%. 1 H NMR (DMSO-d 6 , 400MHz) δ: 0.94(t, J=7.21Hz, 3H), 1.25(d, J=7.22Hz, 6H), 1.76(m, 2H), 2.55(s, 3H), 2.79(t, J=7.24 Hz, 2H), 3.94 (m, 1H), 7.46 (s, 1H), 7.83 (s, 1H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com