Nanometer fluorescence material with nuclear shell structure and preparation method thereof
A technology of fluorescent nanomaterials and core-shell structure, applied in the field of fluorescent nanomaterials with core-shell structure and its preparation, can solve the problems of low crystallinity, achieve good crystallization, convenient operation, and reduce production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
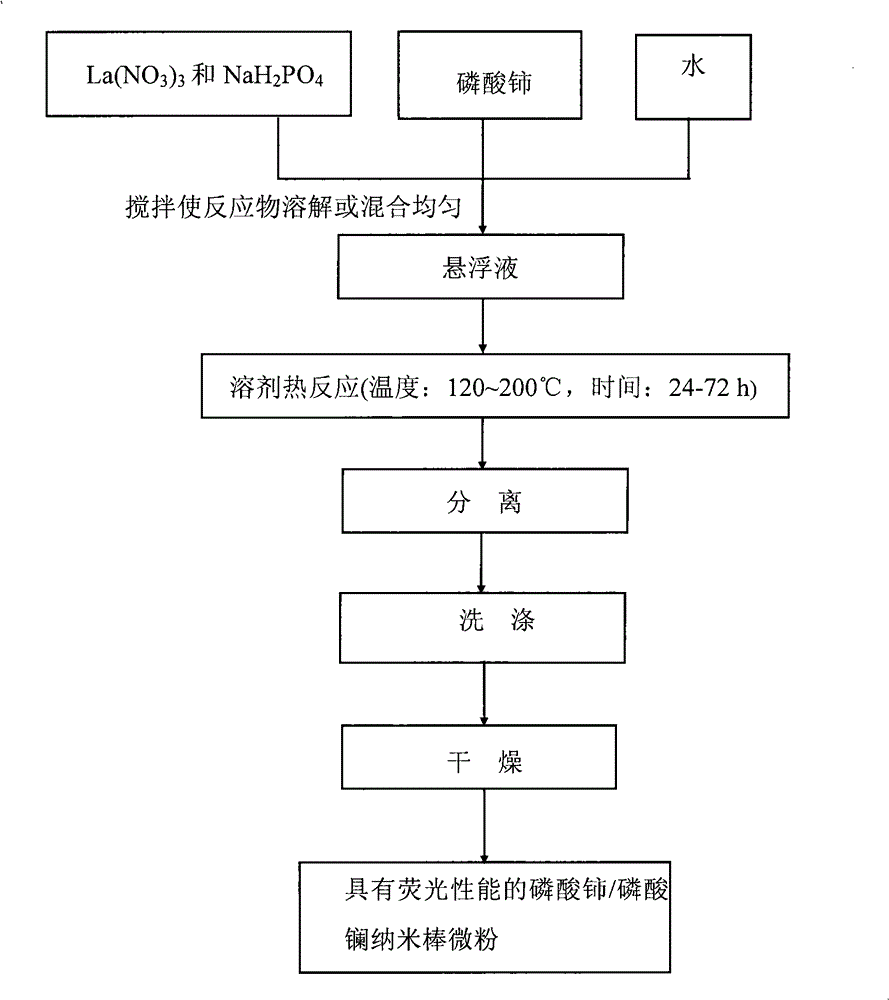
[0045] 1. Preparation of cerium phosphate with monoclinic structure
[0046] Prepare monoclinic cerium phosphate according to the following steps
[0047] 1) 0.434g Ce(NO 3 ) 3 ·6H 2 O and 0.156 g NaH 2 PO 4 2H 2 O was added to 15mL distilled water, stirred evenly, all the obtained suspension A was transferred to a 20ml pressure-resistant reactor, and the reactor was sealed;
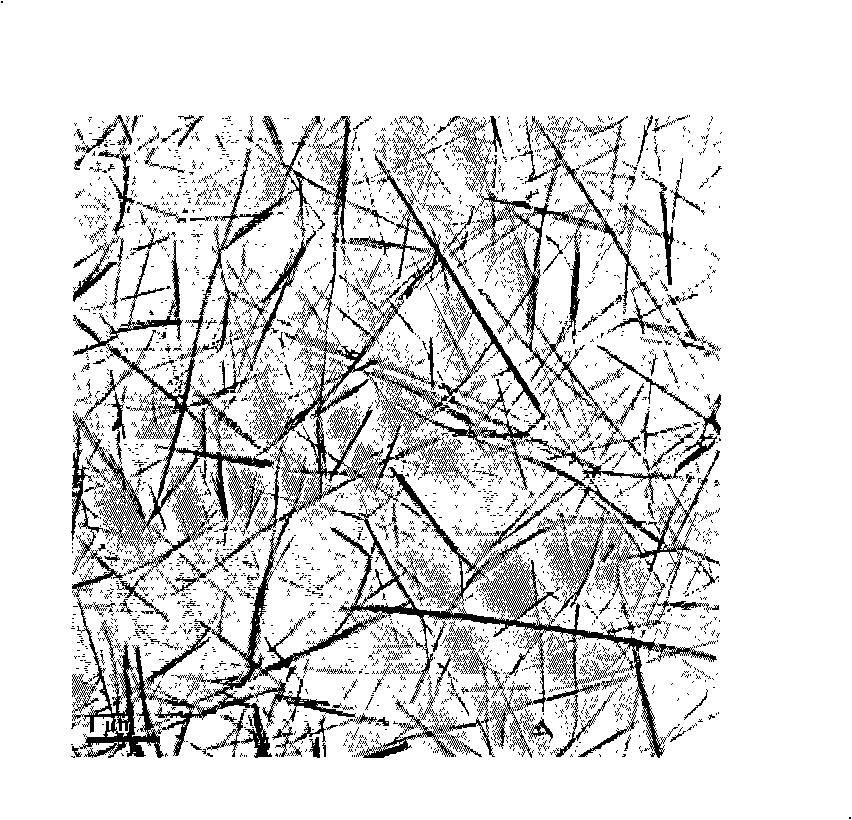
[0048] 2) Put the reaction kettle into an oven and heat it to 200°C, keep it warm for 24 hours, then cool it down to 20°C to obtain the monoclinic CePO of the present invention 4 suspension.
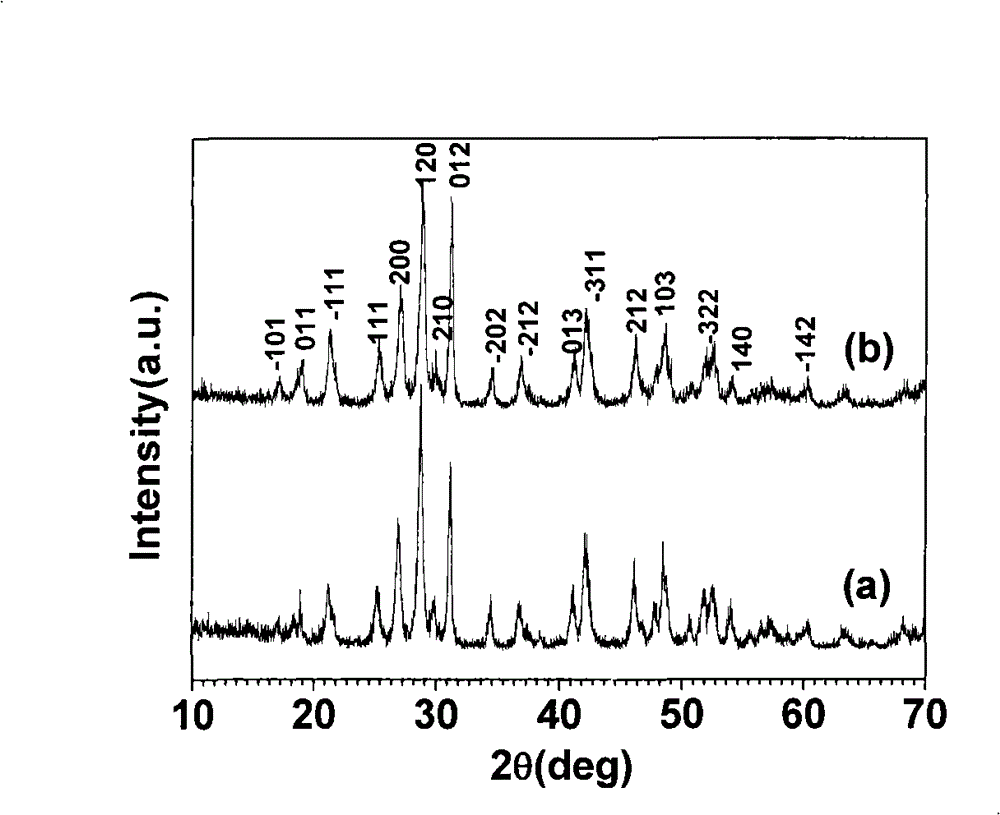
[0049] The prepared cerium phosphate was detected by X-ray diffraction method, and the performance index measurement results are shown in Table 1.
[0050] Fluorescence intensity of cerium phosphate was detected at an excitation wavelength of 280 nm by a Fluorolog-3 fluorescence spectrometer (JOBIN YVON), and the detection results are shown in Table 2.
[0051] figure 2 (a) is the X-ray diffraction spect...
Embodiment 2
[0066] 1. In the process of preparing the monoclinic cerium phosphate solution, except that the reactant is 0.372g CeCl 3 ·7H 2 O and 0.358gNa 2 HPO 4 12H 2 0, temperature of reaction is 230 ℃, and the reaction times is except that 12h, all the other are identical with embodiment 1;
[0067] The X-ray diffraction detection results of the prepared cerium phosphate nanorods are shown in Table 1, and the X-ray diffraction spectrum is completely consistent with the standard JCPDS card (No.32-0199); the fluorescence intensity detection results are shown in Table 2.
[0068] Figure 8 (a) is the fluorescence spectrum of cerium phosphate nanorods.
[0069] 2. In the process of preparing cerium phosphate / lanthanum phosphate nanorods, except that the reactant is 0.185g LaCl 3 ·7H 2 O and 0.179g Na 2 HPO 4 12H 2 O, the reaction temperature is 160°C, and the reaction time is 60h, all the other are the same as in Example 1.
[0070] The fluorescence intensity of the prepared ce...
Embodiment 3
[0074] 1. During the preparation of monoclinic cerium phosphate suspension, except that the reactant is 0.372g CeCl 7H 2 o 3 and 0.380g Na 3 PO 4 12H 2 0, temperature of reaction is 180 ℃, and the reaction times is outside 72h, identical with embodiment 1;
[0075] The X-ray diffraction detection results of the prepared cerium phosphate nanorods are shown in Table 1, and the X-ray diffraction spectrum is completely consistent with the standard JCPDS card (No.32-0199); the fluorescence intensity detection results are shown in Table 2.
[0076] Figure 9 (a) is the fluorescence spectrum of cerium phosphate nanorods.
[0077] 2. In the process of preparing cerium phosphate / lanthanum phosphate nanorods, except that the reactant is 0.158g La(Ac) 3 and 0.190gNa 3 PO 4 12H 2 O, the reaction temperature is 200°C, and the reaction time is 12h, the rest are the same as in Example 1.
[0078] The fluorescence intensity of the prepared cerium phosphate / lanthanum phosphate nanoro...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com