Anthocyanidin identification method
An identification method, anthocyanin technology, applied in the field of identification of natural pigments, can solve the problems of tediousness, very high equipment and technical requirements, etc., and achieve the effect of high precision, convenient rapid preliminary identification, and low equipment and technical requirements
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
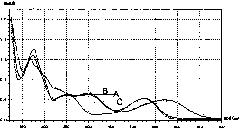
[0014] (1) Take 3 milligrams of cyanidin samples containing an adjacent hydroxyl structure, add 15 milliliters of methanol, and shake well;
[0015] (2) Use a cuvette to take 3 milliliters of the anthocyanin methanol solution in (1), scan it with a UV-visible light spectrophotometer in the range of 220nm to 700nm, use methanol as a reference, and obtain the maximum absorption peak of visible light according to the scanning curve at Around 530nm;
[0016] (3) Take 5 ml of anthocyanin methanol solution prepared in (1), add 3 drops of 5% ACl 3 Take 3 milliliters after the solution is fully shaken, add it to the cuvette and repeat the scanning by the method in (2), according to the scanning curve, the maximum absorption peak of visible light shifts to the long wavelength direction, and moves to around 580nm;
[0017] (4) Take 5 ml of anthocyanin methanol solution prepared in (1), add 3 drops of 5% ACl 3 Shake the solution well, then add 3 drops of 15% HCl solution and shake well...
Embodiment 2
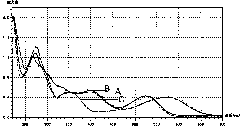
[0019] (1) Take 5 mg of a sample of peony anthocyanin containing an o-hydroxy structure, add 15 ml of methanol, and shake well;
[0020] (2) Use a cuvette to take 3 milliliters of the anthocyanin methanol solution in (1), scan it with a UV-visible light spectrophotometer in the range of 220nm to 700nm, use methanol as a reference, and obtain the maximum absorption peak of visible light according to the scanning curve at Around 530nm;
[0021] (3) Take 5 ml of anthocyanin methanol solution prepared in (1), add 5 drops of 10% ACl 3 Take 3 milliliters after the solution is fully shaken, add it to the cuvette and repeat the scanning by the method in (2), according to the scanning curve, the maximum absorption peak of visible light shifts to the long wavelength direction, and moves to around 580nm;
[0022] (4) Take 5 ml of anthocyanin methanol solution prepared in (1), add 5 drops of 10% ACl 3 Shake the solution well, then add 5 drops of 20% HCl solution and shake well, then tak...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com