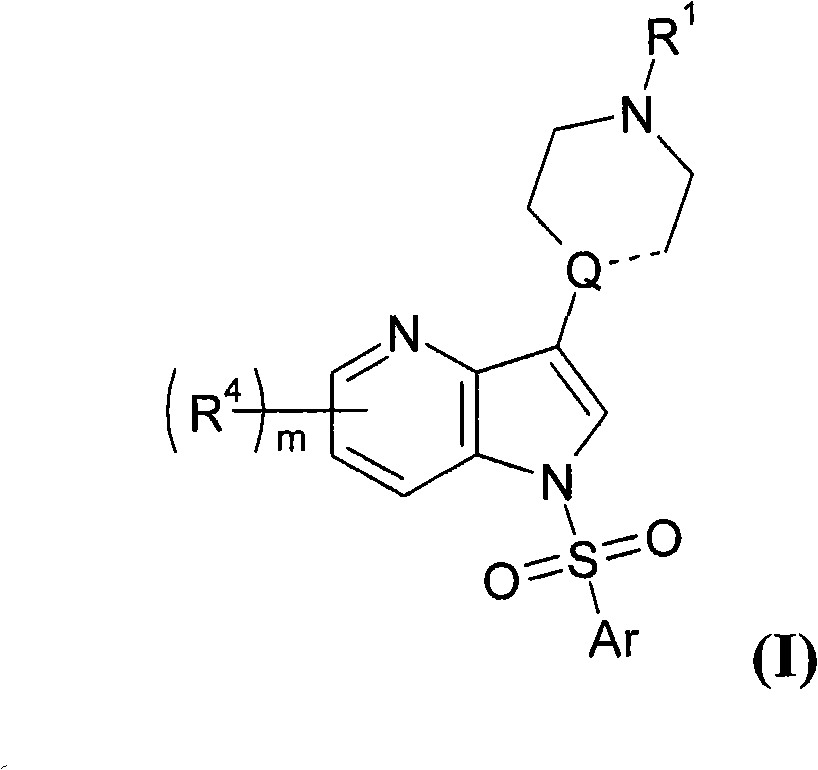
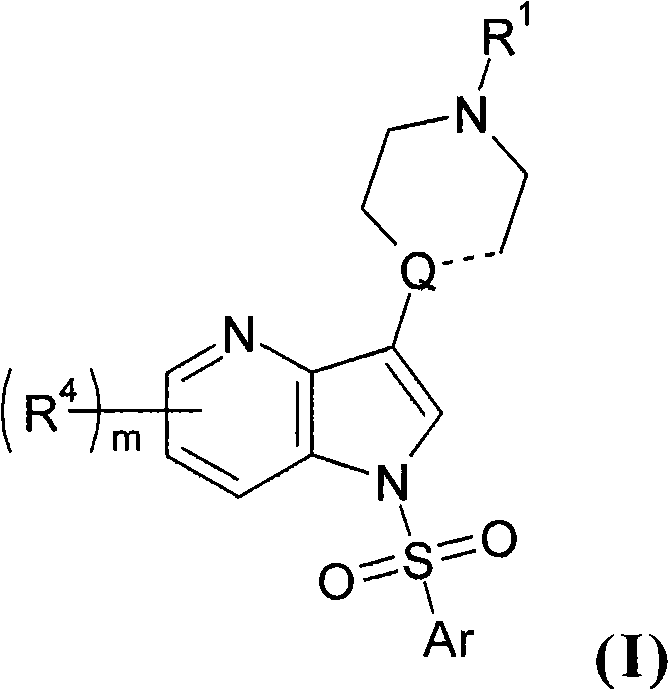
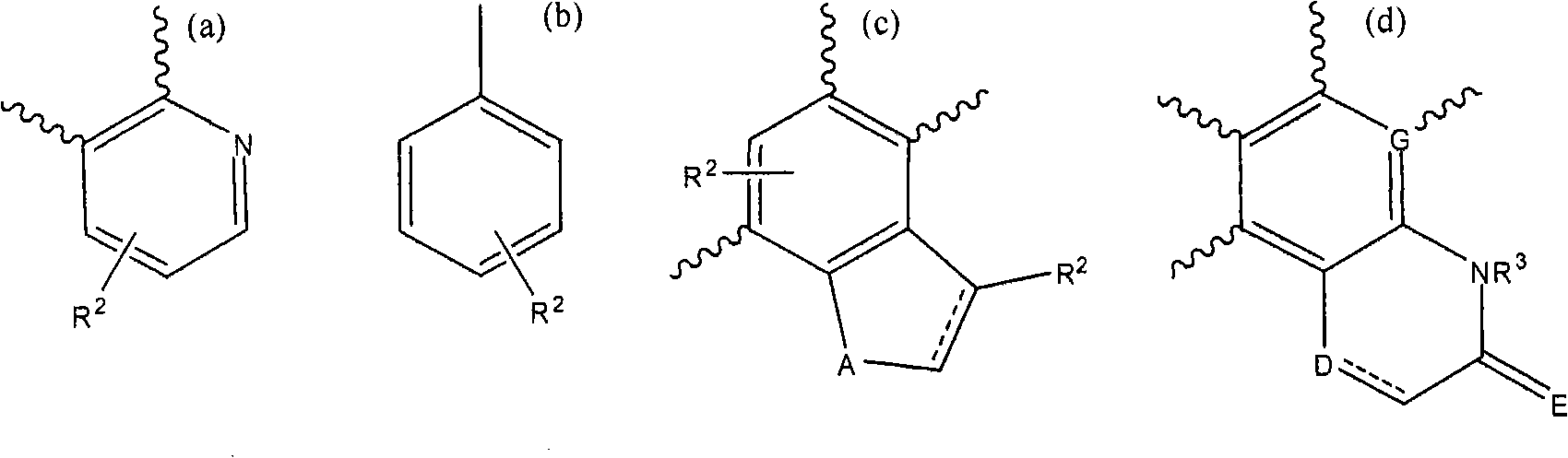
3' substituted compounds having 5-ht6 receptor affinity
A technology of compounds and solvates, applied in the field of compounds with selective 5-HT6 affinity, can solve the problem of selective agonists and antagonists hindering receptor function in vivo research and other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0452] I. Preparation of Sulfonyl Chloride
[0453] The sulfonyl chlorides used herein are commercially available from suppliers such as Sigma-Aldrich, Milwaukee, WI US; Lancaster Synthesis, Windham, NH USA; or Maybridge Chemical Co. Ltd., Cornwall, UK; or can be obtained by methods known in the art or Prepare according to the method outlined below.
[0454] For example, benzenesulfonyl chloride, 2-chlorobenzenesulfonyl chloride, 3-chlorobenzenesulfonyl chloride, 4-chlorobenzenesulfonyl chloride, 2-fluorobenzenesulfonyl chloride, 3-fluorobenzenesulfonyl chloride, 4-fluorobenzenesulfonyl chloride, 2- Methoxybenzenesulfonyl chloride, 3-methoxybenzenesulfonyl chloride, 4-methoxybenzenesulfonyl chloride, 2-difluoromethoxybenzenesulfonyl chloride, 3-difluoromethoxybenzenesulfonyl chloride, 4-di Fluoromethoxybenzenesulfonyl chloride, 2-trifluoromethoxybenzenesulfonyl chloride, 3-trifluoromethoxybenzenesulfonyl chloride, 4-trifluoromethoxybenzenesulfonyl chloride, 3-trifluoromethylb...
Embodiment 1
[0847] Example 1: Synthesis of 3-piperazin-1-yl-1-[(3-pyrrolidin-1-ylphenyl)sulfonyl]-1H-pyrrolo[3,2-b]pyridine (compound 64)
[0848]
[0849] 4-(1H-pyrrolo[3,2-b]pyridin-3-yl)piperazine-1-carboxylic acid tert-butyl ester (112 mg, 0.000369 mol) and tetrahydrofuran (3 mL, 0.03 mol) and N, N-di Methylformamide (3 mL, 0.03 mol) was added to the vial. The material was stirred at 5 °C under nitrogen atmosphere and a 1.0 M solution of sodium bis(trimethylsilyl)amide in tetrahydrofuran (0.44 mL) was added. The reaction was stirred for 10 minutes and a solution of 3-pyrrolidin-1-ylbenzenesulfonyl chloride (163 mg, 0.000665 mol) in tetrahydrofuran (4 mL, 0.05 mol) was added followed by N,N-dimethylethylamine (112 uL , 0.00103mol). The reaction was stirred for 20 minutes and extracted with ethyl acetate, washed with water and brine. The solvent was concentrated in vacuo. The residue was stirred in dichloromethane (5 mL, 0.08 mol), and trifluoroacetic acid (1 mL, 0.01 mol) was ad...
Embodiment 2
[0851] Example 2: 3-{[3-(1,2,3,6-tetrahydropyridin-4-yl)-1H-pyrrolo[3,2-b]pyridin-1 base]sulfonyl}quinoline ( Compound 50) Synthesis
[0852]
[0853] 4-(1H-pyrrolo[3,2-b]pyridin-3-yl)-3,6-dihydropyridine-1(2H)-carboxylic acid tert-butyl ester (99.0 mg, 0.000331mol) was stirred in tetrahydrofuran (3mL, 0.04mol) and N,N-dimethylformamide (3mL, 0.04mol), and a solution of 1.0M sodium bis(trimethylsilyl)amide in tetrahydrofuran was added ( 0.50mL). The reaction was stirred for 30 minutes and added to a mixture containing quinoline-3-sulfonyl chloride hydrochloride (131 mg, 0.000496 mol;) and tetrahydrofuran (3 mL, 0.04 mol) and N,N-dimethyl ethylamine (53.8 uL, 0.000496 mol) in a 1 neck round bottom flask. Gas evolution was observed during the transfer of the azaindole anion solution to the sulfonyl chloride solution (once the base was added to the suspension in THF, it became clear. The reaction was stirred for 30 minutes. The reaction was washed with acetic acid Ethyl et...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com